当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanoscopic spontaneous poration as a precursor to protein-based transport in early protocells
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-14 , DOI: 10.1039/d4cp03979c Tai-You Chu, Chia-Hsuan Lee, Minh Thuy Vo, Ian Liau
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-14 , DOI: 10.1039/d4cp03979c Tai-You Chu, Chia-Hsuan Lee, Minh Thuy Vo, Ian Liau
|
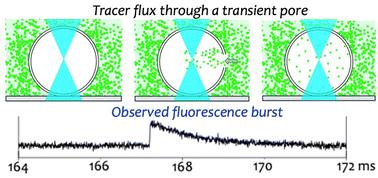
Understanding the mechanisms of material transport in protocells before the emergence of proteins is crucial to uncovering the origins of cellular life. While previous research has demonstrated that direct permeation is a feasible transport mechanism for protocells with fatty acid-based membranes, this process becomes less efficient as membranes evolve to include phospholipids—before the advent of protein transport systems. To address this knowledge gap, we investigated fundamental processes that could have facilitated molecular transport in such protein-free systems. In this study, we identify and characterize nanoscopic transient pores spontaneously forming in phospholipid vesicle membranes, likely driven by osmotic imbalances. We for the first time pinpointed individual pore formation events by observing intermittent fluorescence bursts resulting from the brief influx of fluorescent tracers into the vesicular interior. Kinetic analysis of these burst profiles reveals that these membrane pores possess lifespans of about fourteen milliseconds and radii of around twenty nanometers, suggesting that they are sufficiently large and long-lived to enable the transport of essential nutrients and metabolic products. These findings are confirmed by conventional pore-sizing methods using tracers of various sizes and supported by numerical simulations. Importantly, this transient pore formation does not compromise the integrity of the membrane, nor does it require the participation of proteins or peptides. Our results indicate that spontaneous transient poration provides a viable mechanism for molecular transport through the membrane of primitive cellular entities, offering an alternative to simple diffusion or direct permeation. This study sheds light on potential evolutionary strategies employed by pre-protein protocellular entities to facilitate material transport, contributing to our understanding of the early mechanisms that may have driven the origin of life.
中文翻译:

纳米级自发孔化是早期原始细胞中基于蛋白质的转运的前体
在蛋白质出现之前了解原始细胞中物质运输的机制对于揭示细胞生命的起源至关重要。虽然先前的研究表明,直接渗透是具有脂肪酸基膜的原始细胞的可行运输机制,但随着膜进化到包含磷脂,这一过程的效率会降低——在蛋白质运输系统出现之前。为了解决这一知识差距,我们研究了在这种无蛋白质系统中可能促进分子转运的基本过程。在这项研究中,我们识别和表征了磷脂囊泡膜中自发形成的纳米级瞬时孔,这可能是由渗透失衡驱动的。我们首次通过观察荧光示踪剂短暂流入囊泡内部导致的间歇性荧光爆发来确定单个孔的形成事件。对这些爆发曲线的动力学分析表明,这些膜孔的寿命约为 14 毫秒,半径约为 20 纳米,这表明它们足够大且寿命长,能够运输必需的营养物质和代谢产物。这些发现通过使用各种大小的示踪剂的常规孔径测定方法得到证实,并得到数值模拟的支持。重要的是,这种瞬时孔的形成不会损害膜的完整性,也不需要蛋白质或肽的参与。我们的结果表明,自发瞬时穿孔为分子通过原始细胞实体膜的运输提供了一种可行的机制,为简单的扩散或直接渗透提供了一种替代方案。 这项研究阐明了前蛋白质原细胞实体为促进物质运输而采用的潜在进化策略,有助于我们理解可能驱动生命起源的早期机制。
更新日期:2024-11-14
中文翻译:

纳米级自发孔化是早期原始细胞中基于蛋白质的转运的前体
在蛋白质出现之前了解原始细胞中物质运输的机制对于揭示细胞生命的起源至关重要。虽然先前的研究表明,直接渗透是具有脂肪酸基膜的原始细胞的可行运输机制,但随着膜进化到包含磷脂,这一过程的效率会降低——在蛋白质运输系统出现之前。为了解决这一知识差距,我们研究了在这种无蛋白质系统中可能促进分子转运的基本过程。在这项研究中,我们识别和表征了磷脂囊泡膜中自发形成的纳米级瞬时孔,这可能是由渗透失衡驱动的。我们首次通过观察荧光示踪剂短暂流入囊泡内部导致的间歇性荧光爆发来确定单个孔的形成事件。对这些爆发曲线的动力学分析表明,这些膜孔的寿命约为 14 毫秒,半径约为 20 纳米,这表明它们足够大且寿命长,能够运输必需的营养物质和代谢产物。这些发现通过使用各种大小的示踪剂的常规孔径测定方法得到证实,并得到数值模拟的支持。重要的是,这种瞬时孔的形成不会损害膜的完整性,也不需要蛋白质或肽的参与。我们的结果表明,自发瞬时穿孔为分子通过原始细胞实体膜的运输提供了一种可行的机制,为简单的扩散或直接渗透提供了一种替代方案。 这项研究阐明了前蛋白质原细胞实体为促进物质运输而采用的潜在进化策略,有助于我们理解可能驱动生命起源的早期机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号