当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
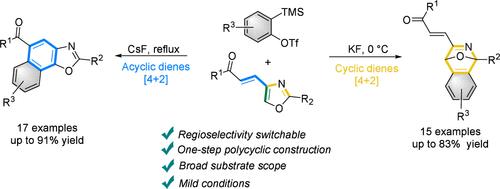
Construction of Naphtho[2,1-d]oxazoles and 1,4-Epoxyisoquinolines via Regioselectivity-Switchable Diels–Alder Reaction of 4-Alkenyloxazoles with Arynes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.joc.4c01919 Ying-Yi Wang, Feng Sha, Yu-Hui Zheng, Xin-Yan Wu
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.joc.4c01919 Ying-Yi Wang, Feng Sha, Yu-Hui Zheng, Xin-Yan Wu
|
A Regioselectivity-switchable Diels–Alder reaction of arynes and 4-alkenyloxazoles was explored. By regulating the reaction temperature and the polarity of the solvent, the reaction activity of aryne was controlled to selectively react with different favorable reaction sites of 4-alkenyloxazoles, providing naphtho[2,1-d]oxazoles and 1,4-epoxyisoquinolines. Density functional theory (DFT) calculations demonstrated that the Diels–Alder reaction on acyclic dienes site needs more energy than the reaction on cyclic dienes to overcome the potential reaction barrier.
中文翻译:

通过 4-烯氧唑与芳烃的区域选择性可切换 Diels-Alder 反应构建萘酚[2,1-d]噁唑和 1,4-环氧异喹啉
探索了芳烃和 4-烯氧唑的 Regioselectivity 可切换 Diels-Alder 反应。通过调节反应温度和溶剂极性,控制芳烃的反应活性,选择性地与 4-烯氧唑类化合物的不同有利反应位点反应,得到萘酚[2,1-d]噁唑类和 1,4-环氧异喹啉类。密度泛函理论 (DFT) 计算表明,非环二烯位点上的 Diels-Alder 反应比环二烯上的反应需要更多的能量来克服潜在的反应障碍。
更新日期:2024-11-14
中文翻译:

通过 4-烯氧唑与芳烃的区域选择性可切换 Diels-Alder 反应构建萘酚[2,1-d]噁唑和 1,4-环氧异喹啉
探索了芳烃和 4-烯氧唑的 Regioselectivity 可切换 Diels-Alder 反应。通过调节反应温度和溶剂极性,控制芳烃的反应活性,选择性地与 4-烯氧唑类化合物的不同有利反应位点反应,得到萘酚[2,1-d]噁唑类和 1,4-环氧异喹啉类。密度泛函理论 (DFT) 计算表明,非环二烯位点上的 Diels-Alder 反应比环二烯上的反应需要更多的能量来克服潜在的反应障碍。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号