当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cascade Cyclization/Annulation of β-Enamino Diketones and o-Phenylenediamine: A Strategy to Access Pyrrole-fused 1,5-Benzodiazepines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.joc.4c02483 Julia Poletto, Julia C. M. Willig, Jeniffer N. A. Camargo, Helio G. Bonacorso, Michael J. V. Silva, Fernanda A. Rosa
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.joc.4c02483 Julia Poletto, Julia C. M. Willig, Jeniffer N. A. Camargo, Helio G. Bonacorso, Michael J. V. Silva, Fernanda A. Rosa
|
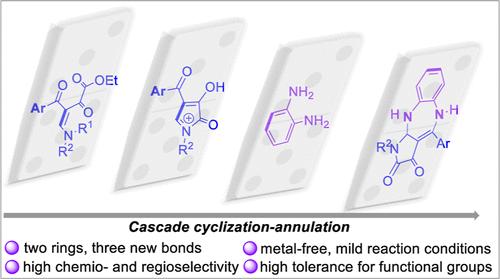
Herein, we introduce an unprecedented cascade reaction for the assembly of pyrrole-fused 1,5-benzodiazepine frameworks. These diverse privileged scaffolds were controllably constructed by intramolecular cyclization of β-enamino diketone, followed by annulation with o-phenylenediamine. The protocol features efficient one-pot cascade cyclization/annulation, performed under simple and mild reaction conditions. The products are obtained in a metal-free manner, in good to excellent yields (65–91%), and represent a fused heterocyclic scaffold that is not yet found in nature.
中文翻译:

β-烯氨基二酮和邻苯二胺的级联环化/环化:获得吡咯融合的 1,5-苯二氮卓类药物的策略
在此,我们介绍了一种前所未有的级联反应,用于吡咯熔融的 1,5-苯二氮卓类框架的组装。这些不同的特权支架是通过 β-烯氨基二酮的分子内环化,然后用邻苯二胺环化来控制构建的。该方案具有高效的一锅级联环化/环化,可在简单和温和的反应条件下进行。这些产品以无金属方式获得,产率从好到极高 (65-91%),代表了自然界中尚未发现的熔融杂环支架。
更新日期:2024-11-14
中文翻译:

β-烯氨基二酮和邻苯二胺的级联环化/环化:获得吡咯融合的 1,5-苯二氮卓类药物的策略
在此,我们介绍了一种前所未有的级联反应,用于吡咯熔融的 1,5-苯二氮卓类框架的组装。这些不同的特权支架是通过 β-烯氨基二酮的分子内环化,然后用邻苯二胺环化来控制构建的。该方案具有高效的一锅级联环化/环化,可在简单和温和的反应条件下进行。这些产品以无金属方式获得,产率从好到极高 (65-91%),代表了自然界中尚未发现的熔融杂环支架。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号