当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of α-Boryl-α-Substituted Allylboronates from Propyne
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.orglett.4c03683 Hiro Takayanagi, Naoki Oku, Ken Yamazaki, Tomoya Miura
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.orglett.4c03683 Hiro Takayanagi, Naoki Oku, Ken Yamazaki, Tomoya Miura
|
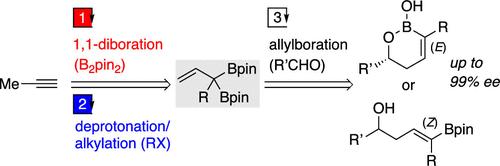
A novel method for the two-step synthesis of α-boryl-α-substituted allylboronates from propyne is described. These allylboronates are prepared by the Co-catalyzed 1,1-diboration reaction of propyne with B2pin2, followed by the base-mediated alkylation reaction of 1,1-di(boryl)propene at the α-position. Computational studies revealed the origins of observed reactivity and selectivity in the base-mediated alkylation reaction. The resulting α-boryl-α-methyl-allylboronate is applied to the allylation of aldehydes, which gives homoallylic alcohols with a trisubstituted alkene moiety.
中文翻译:

Propyne 合成 α-硼基-α-取代的烯丙基硼酸盐
描述了一种从丙炔两步合成 α-硼基-α-取代烯丙基硼酸盐的新方法。这些烯丙基硼酸盐是通过丙炔与 B2针2 的共催化 1,1-二硼化反应制备的,然后是 1,1-二(硼基)丙烯在 α 位的碱介导的烷基化反应。计算研究揭示了在碱基介导的烷基化反应中观察到的反应性和选择性的来源。所得的 α-硼基-α-甲基烯丙基硼酸酯被应用于醛的烯丙基化,得到具有三取代烯烃部分的均烯丙基醇。
更新日期:2024-11-14
中文翻译:

Propyne 合成 α-硼基-α-取代的烯丙基硼酸盐
描述了一种从丙炔两步合成 α-硼基-α-取代烯丙基硼酸盐的新方法。这些烯丙基硼酸盐是通过丙炔与 B2针2 的共催化 1,1-二硼化反应制备的,然后是 1,1-二(硼基)丙烯在 α 位的碱介导的烷基化反应。计算研究揭示了在碱基介导的烷基化反应中观察到的反应性和选择性的来源。所得的 α-硼基-α-甲基烯丙基硼酸酯被应用于醛的烯丙基化,得到具有三取代烯烃部分的均烯丙基醇。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号