当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brook-Oxidation Reaction of Acylsilanes: General Access to α-Ketoamides and α-Ketothioamides
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.orglett.4c03889 Dan-Ni Yang, Ya-Nan Du, Peng Wang, Man-Yi Han
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.orglett.4c03889 Dan-Ni Yang, Ya-Nan Du, Peng Wang, Man-Yi Han
|
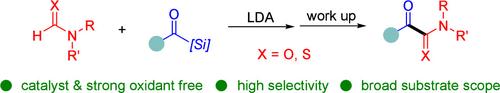
A novel chemoselective Brook-oxidation reaction of acylsilanes initiated by the carbamoyl anion has been successfully developed for the first time. This method enables the synthesis of diverse α-ketoamides and α-ketothioamides under transition metal-free and strong oxidant-free conditions with high yields and high chemoselectivity. It also demonstrates tolerance toward a wide range of functional groups. The synthetic utility of this process is underscored by its successful application in the synthesis of an orexin receptor antagonist from acylsilane, highlighting its potential for the development of novel therapeutic agents and further exploration in synthetic chemistry.
中文翻译:

酰基硅烷的 Brook 氧化反应:α-酮酰胺和 α-酮硫酰胺的一般可及性
由氨基甲酰阴离子引发的酰基硅烷的新型化学选择性 Brook 氧化反应已首次成功开发。该方法能够在无过渡金属和无强氧化剂的条件下合成多种 α-酮酰胺和 α-酮硫酰胺,产量高,化学选择性高。它还显示出对各种官能团的耐受性。该工艺在酰基硅烷合成食欲素受体拮抗剂的成功应用强调了该工艺的合成效用,突出了其开发新型治疗剂和进一步探索合成化学的潜力。
更新日期:2024-11-14
中文翻译:

酰基硅烷的 Brook 氧化反应:α-酮酰胺和 α-酮硫酰胺的一般可及性
由氨基甲酰阴离子引发的酰基硅烷的新型化学选择性 Brook 氧化反应已首次成功开发。该方法能够在无过渡金属和无强氧化剂的条件下合成多种 α-酮酰胺和 α-酮硫酰胺,产量高,化学选择性高。它还显示出对各种官能团的耐受性。该工艺在酰基硅烷合成食欲素受体拮抗剂的成功应用强调了该工艺的合成效用,突出了其开发新型治疗剂和进一步探索合成化学的潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号