当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanochemical Decarbonylative Cross-Coupling of Amides via Cooperative Catalysis and Triple C–N/C–C/C–H Activation
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-13 , DOI: 10.1021/acssuschemeng.4c05179 Jin Zhang, Jiaojiao Zhang, Wenxuan Yan, Sijie Zhou, Yangmin Ma, Michal Szostak
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-13 , DOI: 10.1021/acssuschemeng.4c05179 Jin Zhang, Jiaojiao Zhang, Wenxuan Yan, Sijie Zhou, Yangmin Ma, Michal Szostak
|
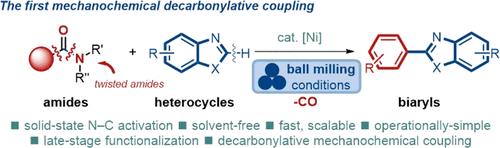
Mechanochemical solvent-less cross-couplings have emerged as a powerful frontier in the formation of carbon–carbon and carbon–heteroatom bonds under sustainable conditions. However, despite considerable progress, mechanochemical decarbonylative cross-couplings have been unexplored. Herein, we report the first decarbonylative mechanochemical cross-coupling for the heteroarylation of amides by a triple C–N/C–C/C–H activation. The catalytic system exploits cooperative Ni/Cu catalysis to simultaneously activate the amide N–C(O) and the heterocycle C–H bonds, resulting in a highly chemoselective coupling. The reaction is characterized by a broad scope of the amide and the heterocyclic component to furnish heterobiaryls, which are among the most privileged scaffolds in organic synthesis. We demonstrate that decarbonylative mechanochemical cross-couplings will provide novel access to a range of valuable products that are among the most common structures in organic chemistry.
中文翻译:

通过协同催化和三重 C-N/C-C/C-H 活化实现酰胺的机械化学脱羰基交叉偶联
机械化学无溶剂交叉偶联已成为在可持续条件下形成碳-碳和碳-杂原子键的强大前沿。然而,尽管取得了相当大的进展,但机械化学脱羰基交叉偶联仍未得到探索。在此,我们报道了通过三重 C-N/C-C/C-H 活化实现酰胺杂芳化的第一个脱羰基机械化学交叉偶联。该催化系统利用协同 Ni/Cu 催化同时激活酰胺 N-C(O) 和杂环 C-H 键,从而产生高化学选择性偶联。该反应的特点是酰胺和杂环组分的广泛范围以提供异联芳基,这是有机合成中最优越的支架之一。我们证明,脱羰基机械化学交叉偶联将为一系列有价值的产品提供新的途径,这些产品是有机化学中最常见的结构之一。
更新日期:2024-11-14
中文翻译:

通过协同催化和三重 C-N/C-C/C-H 活化实现酰胺的机械化学脱羰基交叉偶联
机械化学无溶剂交叉偶联已成为在可持续条件下形成碳-碳和碳-杂原子键的强大前沿。然而,尽管取得了相当大的进展,但机械化学脱羰基交叉偶联仍未得到探索。在此,我们报道了通过三重 C-N/C-C/C-H 活化实现酰胺杂芳化的第一个脱羰基机械化学交叉偶联。该催化系统利用协同 Ni/Cu 催化同时激活酰胺 N-C(O) 和杂环 C-H 键,从而产生高化学选择性偶联。该反应的特点是酰胺和杂环组分的广泛范围以提供异联芳基,这是有机合成中最优越的支架之一。我们证明,脱羰基机械化学交叉偶联将为一系列有价值的产品提供新的途径,这些产品是有机化学中最常见的结构之一。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号