Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TC-Sniffer: A Transformer-CNN Bibranch Framework Leveraging Auxiliary VOCs for Few-Shot UBC Diagnosis via Electronic Noses
ACS Sensors ( IF 8.2 ) Pub Date : 2024-11-13 , DOI: 10.1021/acssensors.4c02073 Yingying Jian, Nan Zhang, Yunzhe Bi, Xiyang Liu, Jinhai Fan, Weiwei Wu, Taoping Liu
ACS Sensors ( IF 8.2 ) Pub Date : 2024-11-13 , DOI: 10.1021/acssensors.4c02073 Yingying Jian, Nan Zhang, Yunzhe Bi, Xiyang Liu, Jinhai Fan, Weiwei Wu, Taoping Liu
|
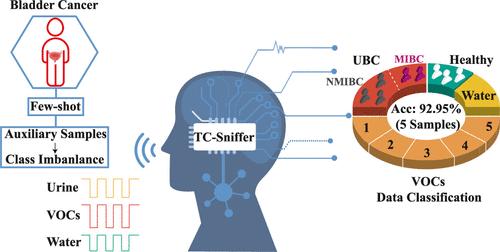
Utilizing electronic noses (e-noses) with pattern recognition algorithms offers a promising noninvasive method for the early detection of urinary bladder cancer (UBC). However, limited clinical samples often hinder existing artificial intelligence (AI)-assisted diagnosis. This paper proposes TC-Sniffer, a novel bibranch framework for few-shot UBC diagnosis, leveraging easily obtainable UBC-related volatile organic components (VOCs) as auxiliary classification categories. These VOCs are biomarkers of UBC, helping the model learn more UBC-specific features, reducing overfitting in small sample scenarios, and reflecting the imbalanced distribution of clinical samples. TC-Sniffer employs intensity-based augmentation to address small sample size issues and focal loss to alleviate model bias due to the class imbalance caused by auxiliary VOCs. The architecture combines transformers and temporal convolutional neural networks to capture long- and short-range dependencies, achieving comprehensive representation learning. Additionally, feature-level constraints further enhance the learning of distinctive features for each class. Experimental results using e-nose data collected from a custom-designed sensor array show that TC-Sniffer significantly surpasses existing approaches, achieving a mean accuracy of 92.95% with only five UBC training samples. Moreover, the fine-grained classification results show that the model can distinguish between nonmuscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC), both of which are subtypes of UBC. The superior performance of TC-Sniffer highlights its potential for timely and accurate cancer diagnosis in challenging clinical settings.
中文翻译:

TC-Sniffer:一种 Transformer-CNN 双分支框架,利用辅助 VOC 通过电子鼻进行少镜头 UBC 诊断
利用电子鼻 (e-noses) 和模式识别算法为膀胱癌 (UBC) 的早期检测提供了一种很有前途的无创方法。然而,有限的临床样本往往会阻碍现有的人工智能 (AI) 辅助诊断。本文提出了 TC-Sniffer,这是一种用于小样本 UBC 诊断的新型双分支框架,利用容易获得的 UBC 相关挥发性有机成分 (VOCs) 作为辅助分类类别。这些 VOC 是 UBC 的生物标志物,帮助模型学习更多 UBC 特定特征,减少小样本场景中的过拟合,并反映临床样本的不平衡分布。TC-Sniffer 采用基于强度的增强来解决小样本量问题和焦点损失,以减轻由于辅助 VOC 引起的类别不平衡而导致的模型偏差。该架构结合了 transformer 和时间卷积神经网络来捕获长距离和短距离依赖关系,实现全面的表示学习。此外,特征级约束进一步增强了每个类对独特特征的学习。使用从定制设计的传感器阵列收集的电子鼻数据的实验结果表明,TC-Sniffer 显着超越了现有方法,仅用 5 个 UBC 训练样本就实现了 92.95% 的平均准确率。此外,细粒度分类结果表明,该模型可以区分非肌层浸润性膀胱癌 (NMIBC) 和肌层浸润性膀胱癌 (MIBC),两者都是 UBC 的亚型。TC-Sniffer 的卓越性能凸显了其在具有挑战性的临床环境中及时准确诊断癌症的潜力。
更新日期:2024-11-14
中文翻译:

TC-Sniffer:一种 Transformer-CNN 双分支框架,利用辅助 VOC 通过电子鼻进行少镜头 UBC 诊断
利用电子鼻 (e-noses) 和模式识别算法为膀胱癌 (UBC) 的早期检测提供了一种很有前途的无创方法。然而,有限的临床样本往往会阻碍现有的人工智能 (AI) 辅助诊断。本文提出了 TC-Sniffer,这是一种用于小样本 UBC 诊断的新型双分支框架,利用容易获得的 UBC 相关挥发性有机成分 (VOCs) 作为辅助分类类别。这些 VOC 是 UBC 的生物标志物,帮助模型学习更多 UBC 特定特征,减少小样本场景中的过拟合,并反映临床样本的不平衡分布。TC-Sniffer 采用基于强度的增强来解决小样本量问题和焦点损失,以减轻由于辅助 VOC 引起的类别不平衡而导致的模型偏差。该架构结合了 transformer 和时间卷积神经网络来捕获长距离和短距离依赖关系,实现全面的表示学习。此外,特征级约束进一步增强了每个类对独特特征的学习。使用从定制设计的传感器阵列收集的电子鼻数据的实验结果表明,TC-Sniffer 显着超越了现有方法,仅用 5 个 UBC 训练样本就实现了 92.95% 的平均准确率。此外,细粒度分类结果表明,该模型可以区分非肌层浸润性膀胱癌 (NMIBC) 和肌层浸润性膀胱癌 (MIBC),两者都是 UBC 的亚型。TC-Sniffer 的卓越性能凸显了其在具有挑战性的临床环境中及时准确诊断癌症的潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号