当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Exploration of N-Heterocyclic Carbene Boranes as the Hydrogen Atom Transfer Reagent in Selective Hydrodefluorination Reactions
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-13 , DOI: 10.1021/acscatal.4c05092 Amit K. Jaiswal, Bastian Bjerkem Skjelstad, Satoshi Maeda, Dennis Chung-Yang Huang
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-13 , DOI: 10.1021/acscatal.4c05092 Amit K. Jaiswal, Bastian Bjerkem Skjelstad, Satoshi Maeda, Dennis Chung-Yang Huang
|
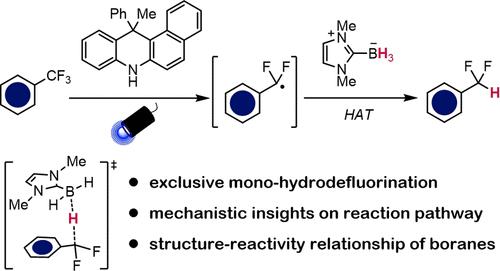
In the modern era of organic synthesis, mechanisms centered on radical intermediates have become increasingly impactful. Among all of these, hydrogen atom transfer (HAT) represents one of the most fundamental chemical reaction steps and has found applications in designing practical transformations. Herein, we present a detailed case study on selective hydrodefluorination of trifluoromethylarenes utilizing N-heterocyclic carbene boranes (NHC-boranes) as the hydrogen atom donor. Under the optimal conditions featuring an acridine-based photocatalyst, complete selectivity for mono-hydrodefluorination was achieved across a wide array of substrates. Comprehensive mechanistic studies combining experimental and computational approaches disproved a chain process involving fluorine atom transfer but rather pointed to a HAT non-chain mechanism, where the key step involves the difluorobenzylic radical abstracting a hydrogen atom from the NHC-borane to generate a boryl radical in a polarity-matched fashion. Evaluation of a selection of Lewis base-ligated boranes revealed molecular descriptors critical to the outcomes of this reaction, and a classification model was built to explain the structure–reactivity relationship and how various elementary steps can be influenced. These results collectively provide valuable information for future reaction design to increase the utility of boranes in organic radical chemistry.
中文翻译:

N-杂环卡宾硼烷作为选择性加氢脱氟反应中氢原子转移试剂的机理探索
在有机合成的现代时代,以自由基中间体为中心的机制已经变得越来越有影响力。其中,氢原子转移 (HAT) 是最基本的化学反应步骤之一,并已应用于设计实际转化。在此,我们提出了一个详细的案例研究,即使用 N-杂环卡宾硼烷 (NHC-硼烷) 作为氢原子供体对三氟甲基芳烃进行选择性加氢脱氟。在以吖啶基光催化剂为特色的最佳条件下,在各种基材上实现了单氢脱氟的完全选择性。结合实验和计算方法的综合机理研究反驳了涉及氟原子转移的链过程,而是指出了 HAT 非链机制,其中关键步骤涉及二氟苄基从 NHC-硼烷中提取氢原子,以极性匹配的方式生成硼基自由基。对一系列 Lewis 碱基连接的硼烷的评估揭示了对该反应结果至关重要的分子描述符,并建立了一个分类模型来解释结构-反应性关系以及各种基本步骤如何受到影响。这些结果共同为未来的反应设计提供了有价值的信息,以提高硼烷在有机自由基化学中的实用性。
更新日期:2024-11-14
中文翻译:

N-杂环卡宾硼烷作为选择性加氢脱氟反应中氢原子转移试剂的机理探索
在有机合成的现代时代,以自由基中间体为中心的机制已经变得越来越有影响力。其中,氢原子转移 (HAT) 是最基本的化学反应步骤之一,并已应用于设计实际转化。在此,我们提出了一个详细的案例研究,即使用 N-杂环卡宾硼烷 (NHC-硼烷) 作为氢原子供体对三氟甲基芳烃进行选择性加氢脱氟。在以吖啶基光催化剂为特色的最佳条件下,在各种基材上实现了单氢脱氟的完全选择性。结合实验和计算方法的综合机理研究反驳了涉及氟原子转移的链过程,而是指出了 HAT 非链机制,其中关键步骤涉及二氟苄基从 NHC-硼烷中提取氢原子,以极性匹配的方式生成硼基自由基。对一系列 Lewis 碱基连接的硼烷的评估揭示了对该反应结果至关重要的分子描述符,并建立了一个分类模型来解释结构-反应性关系以及各种基本步骤如何受到影响。这些结果共同为未来的反应设计提供了有价值的信息,以提高硼烷在有机自由基化学中的实用性。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号