当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption Structure–Activity Correlation in the Photocatalytic Chemistry of Methanol and Water on TiO2(110)
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.accounts.4c00578 Shucai Xia, Tianjun Wang, Zefeng Ren, Xueming Yang, Qing Guo, Chuanyao Zhou
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.accounts.4c00578 Shucai Xia, Tianjun Wang, Zefeng Ren, Xueming Yang, Qing Guo, Chuanyao Zhou
|
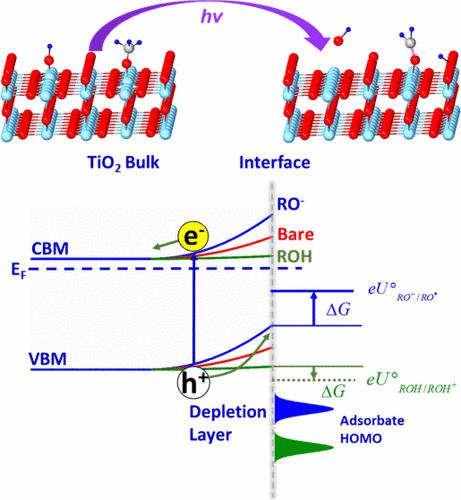
Photocatalysis, a process involving light absorption (band gap excitation), charge separation, interfacial charge transfer, and surface redox reactions, has attracted intensive attention because of the potential applications in solar to fuel conversion. Despite the great efforts devoted to the design of materials and optimization of charge separation and overall efficiency, the molecular mechanism of photocatalytic reactions, for example, water oxidation, is still unclear, mainly because of the complexity of powder catalysts and the aqueous environment which prevent the direct experimental detection of adsorption sites, surface species, and charge/energy transfer dynamics. Without direct evidence, the charge transfer and elementary reaction steps remain elusive, and misleading conclusions are sometimes drawn. For instance, the positively charged 5-fold coordinated Ti sites (Ti5cs) on TiO2 surfaces are argued to propel holes and therefore cannot be active sites for oxidative reactions, regardless of the demonstration by scanning tunneling microscopy (STM). Direct site-specific measurements are thus highly demanded. Surface science studies, which rely on well-defined single crystals and ultrahigh vacuum based techniques, can identify the active sites and active species at the catalyst surfaces and measure the interfacial electronic structure and energy of desorbing species for charge transfer analysis, providing direct evidence for investigating the photocatalytic reaction mechanism at the molecular level.
中文翻译:

TiO2 上甲醇和水光催化化学中的吸附构效相关性(110)
光催化是一种涉及光吸收(带隙激发)、电荷分离、界面电荷转移和表面氧化还原反应的过程,由于在太阳能到燃料转换中的潜在应用而受到广泛关注。尽管在材料设计、电荷分离和整体效率的优化方面投入了大量精力,但光催化反应(例如水氧化)的分子机制仍然不清楚,主要是因为粉末催化剂的复杂性和水环境阻碍了直接实验检测吸附位点、表面种类和电荷/能量转移动力学。如果没有直接证据,电荷转移和基本反应步骤仍然难以捉摸,有时会得出误导性的结论。例如,TiO2 表面上带正电的 5 倍配位 Ti 位点 (Ti5cs) 被认为会推动空穴,因此无论扫描隧道显微镜 (STM) 如何证明,都不能成为氧化反应的活性位点。因此,直接进行现场特定测量是非常必要的。表面科学研究依赖于定义明确的单晶和基于超高真空的技术,可以识别催化剂表面的活性位点和活性物质,并测量脱附物质的界面电子结构和能量以进行电荷转移分析,为在分子水平上研究光催化反应机理提供直接证据。
更新日期:2024-11-13
中文翻译:

TiO2 上甲醇和水光催化化学中的吸附构效相关性(110)
光催化是一种涉及光吸收(带隙激发)、电荷分离、界面电荷转移和表面氧化还原反应的过程,由于在太阳能到燃料转换中的潜在应用而受到广泛关注。尽管在材料设计、电荷分离和整体效率的优化方面投入了大量精力,但光催化反应(例如水氧化)的分子机制仍然不清楚,主要是因为粉末催化剂的复杂性和水环境阻碍了直接实验检测吸附位点、表面种类和电荷/能量转移动力学。如果没有直接证据,电荷转移和基本反应步骤仍然难以捉摸,有时会得出误导性的结论。例如,TiO2 表面上带正电的 5 倍配位 Ti 位点 (Ti5cs) 被认为会推动空穴,因此无论扫描隧道显微镜 (STM) 如何证明,都不能成为氧化反应的活性位点。因此,直接进行现场特定测量是非常必要的。表面科学研究依赖于定义明确的单晶和基于超高真空的技术,可以识别催化剂表面的活性位点和活性物质,并测量脱附物质的界面电子结构和能量以进行电荷转移分析,为在分子水平上研究光催化反应机理提供直接证据。

































 京公网安备 11010802027423号
京公网安备 11010802027423号