当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Tetrafluoroethylene-Mediated Ring-Closing Metathesis: Enabling Unfavored Reactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-13 , DOI: 10.1021/jacs.4c10509 Midori Akiyama, Yuki Amabe, Masafumi Sugiyama, Kanami Sugiyama, Tim Gatzenmeier, Takashi Okazoe
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-13 , DOI: 10.1021/jacs.4c10509 Midori Akiyama, Yuki Amabe, Masafumi Sugiyama, Kanami Sugiyama, Tim Gatzenmeier, Takashi Okazoe
|
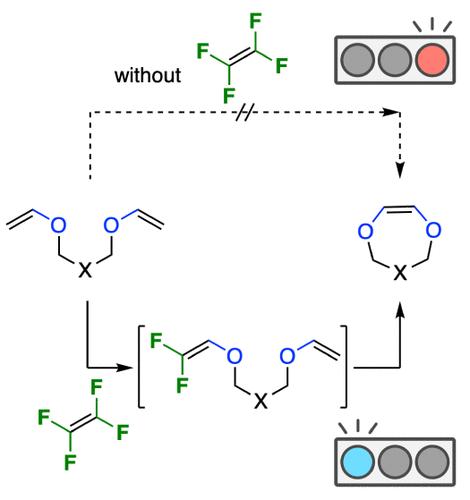
While ring-closing metathesis (RCM) is a powerful method for constructing medium and large cyclic alkenes, its application to the synthesis of heterocycles faces considerable limitations. For instance, RCM of divinyloxyalkanes does not proceed under the conventional conditions of RCM. The challenge lies in the formation of stable Fischer-type carbene intermediates with heteroatom(s) bound to the carbene carbon, impeding subsequent metathesis. In the present research, we develop a novel reaction system termed “tetrafluoroethylene (TFE)-mediated RCM”, which enables the RCM of divinyloxyalkanes. In this system, a divinyloxyalkane and a TFE molecule are converted to the corresponding ring-closing product (cyclic 1,2-dioxyethene) and two molecules of 1,1-difluoroethylene. This method facilitates the construction of six- to eight-membered rings with various functional groups. The addition of TFE has two key effects: thermodynamically, it renders the entire reaction exergonic, while kinetically, it ensures that all ruthenium–carbene intermediates in the catalytic cycle are Fischer-type.
中文翻译:

四氟乙烯介导的闭环复分解:实现不利的反应
虽然闭环复分解 (RCM) 是构建中大型环烯烃的强大方法,但其在杂环合成中的应用面临相当大的限制。例如,二乙烯氧基烷烃的 RCM 不在 RCM 的常规条件下进行。挑战在于形成稳定的 Fischer 型卡宾中间体,杂原子与卡宾碳结合,阻碍随后的复分解。在本研究中,我们开发了一种称为“四氟乙烯 (TFE) 介导的 RCM”的新型反应系统,它使二乙烯氧烷烃的 RCM 成为可能。在该系统中,一个二乙烯氧基烷烃和一个 TFE 分子被转化为相应的闭环产物(环状 1,2-二氧乙烯)和两个 1,1-二氟乙烯分子。这种方法有助于构建具有各种官能团的六到八元环。TFE 的添加有两个关键影响:在热力学上,它使整个反应处于放能状态,而在动力学上,它确保催化循环中的所有钌-卡宾中间体都是 Fischer 型的。
更新日期:2024-11-14
中文翻译:

四氟乙烯介导的闭环复分解:实现不利的反应
虽然闭环复分解 (RCM) 是构建中大型环烯烃的强大方法,但其在杂环合成中的应用面临相当大的限制。例如,二乙烯氧基烷烃的 RCM 不在 RCM 的常规条件下进行。挑战在于形成稳定的 Fischer 型卡宾中间体,杂原子与卡宾碳结合,阻碍随后的复分解。在本研究中,我们开发了一种称为“四氟乙烯 (TFE) 介导的 RCM”的新型反应系统,它使二乙烯氧烷烃的 RCM 成为可能。在该系统中,一个二乙烯氧基烷烃和一个 TFE 分子被转化为相应的闭环产物(环状 1,2-二氧乙烯)和两个 1,1-二氟乙烯分子。这种方法有助于构建具有各种官能团的六到八元环。TFE 的添加有两个关键影响:在热力学上,它使整个反应处于放能状态,而在动力学上,它确保催化循环中的所有钌-卡宾中间体都是 Fischer 型的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号