当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium-Substituted Polyoxoanion Serves as Redox Shuttle and Intermediate Stabilizer in Selective Electrooxidation of Ethylene to Ethylene Glycol
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-13 , DOI: 10.1021/jacs.4c11891 Jiaqi Yu, Charles Bruce Musgrave, III, Qiucheng Chen, Yi Yang, Cong Tian, Xiaobing Hu, Guangcan Su, Heejong Shin, Weiyan Ni, Xinqi Chen, Pengfei Ou, Yuan Liu, Neil M. Schweitzer, Debora Motta Meira, Vinayak P. Dravid, William A. Goddard, III, Ke Xie, Edward H. Sargent
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-13 , DOI: 10.1021/jacs.4c11891 Jiaqi Yu, Charles Bruce Musgrave, III, Qiucheng Chen, Yi Yang, Cong Tian, Xiaobing Hu, Guangcan Su, Heejong Shin, Weiyan Ni, Xinqi Chen, Pengfei Ou, Yuan Liu, Neil M. Schweitzer, Debora Motta Meira, Vinayak P. Dravid, William A. Goddard, III, Ke Xie, Edward H. Sargent
|
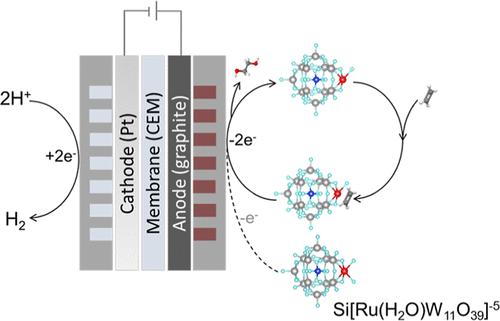
The high carbon intensity of present-day ethylene glycol (EG) production motivates interest in electrifying ethylene oxidation. Noting poor kinetics in prior reports of the organic electrooxidation of small hydrocarbons, we explored the design of mediators that activate and simultaneously stabilize light alkenes. A ruthenium-substituted polyoxometalate (Ru-POM, {Si[Ru(H2O)W11O39]}5–) achieves 82% faradaic efficiency in EG production at 100 mA/cm2 under ambient conditions. Via the union of in situ spectroscopic techniques, electrochemical studies, and density functional theory calculations, we find evidence of a two-step oxidation mechanism: Ru-POM first undergoes electrochemical oxidation to the high valent state, activating ethylene via partial oxidation and forming an intermediate complex; this intermediate complex then migrates to the anode where it undergoes further oxidation to produce EG. The Ru-POM-mediated electrocatalytic system reduces the projected energy consumption required in EG production, requiring 9 GJ per ton of EG (and accompanied by 0.04 ton H2 coproduction), compared to 20–30 GJ/ton in typical prior processes.
中文翻译:

钌取代的聚氧阴离子在乙烯选择性电氧化为乙二醇中用作氧化还原穿梭和中间稳定剂
当今乙二醇 (EG) 生产的高碳强度激发了人们对乙烯氧化电气化的兴趣。注意到先前关于小碳氢化合物有机电氧化的动力学较差,我们探索了激活并同时稳定轻烯烃的介质的设计。在环境条件下,钌取代的多金属氧酸盐 (Ru-POM, {Si[Ru(H2O)W11O39]}5–) 在 100 mA/cm2 的 EG 生产中实现了 82% 的法拉第效率。通过原位光谱技术、电化学研究和密度泛函理论计算的结合,我们发现了两步氧化机制的证据:Ru-POM 首先经历电化学氧化成高价态,通过部分氧化活化乙烯并形成中间络合物;然后,这种中间复合物迁移到阳极,在那里进一步氧化以产生 EG。Ru-POM 介导的电催化系统降低了 EG 生产所需的预计能耗,与典型的先前工艺中的 20-30 GJ/吨相比,每吨 EG 需要 9 GJ(并伴随着 0.04 吨 H2 共生产)。
更新日期:2024-11-14
中文翻译:

钌取代的聚氧阴离子在乙烯选择性电氧化为乙二醇中用作氧化还原穿梭和中间稳定剂
当今乙二醇 (EG) 生产的高碳强度激发了人们对乙烯氧化电气化的兴趣。注意到先前关于小碳氢化合物有机电氧化的动力学较差,我们探索了激活并同时稳定轻烯烃的介质的设计。在环境条件下,钌取代的多金属氧酸盐 (Ru-POM, {Si[Ru(H2O)W11O39]}5–) 在 100 mA/cm2 的 EG 生产中实现了 82% 的法拉第效率。通过原位光谱技术、电化学研究和密度泛函理论计算的结合,我们发现了两步氧化机制的证据:Ru-POM 首先经历电化学氧化成高价态,通过部分氧化活化乙烯并形成中间络合物;然后,这种中间复合物迁移到阳极,在那里进一步氧化以产生 EG。Ru-POM 介导的电催化系统降低了 EG 生产所需的预计能耗,与典型的先前工艺中的 20-30 GJ/吨相比,每吨 EG 需要 9 GJ(并伴随着 0.04 吨 H2 共生产)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号