当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Global Profiling of Protein Phosphorylation, Acetylation, and β-Hydroxybutyrylation in Nannochloropsis oceanica
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.jafc.4c05869 Lingyu Ouyang, Wuxin You, Ansgar Poetsch, Li Wei
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.jafc.4c05869 Lingyu Ouyang, Wuxin You, Ansgar Poetsch, Li Wei
|
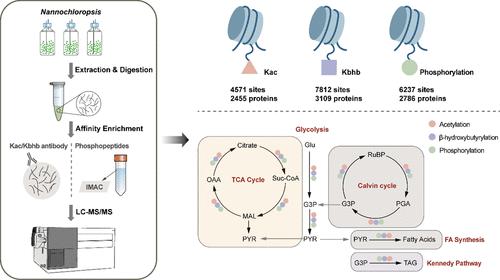
Protein post-translational modifications (PTMs) regulate protein functions but remain poorly characterized in Nannochloropsis. This study examined three PTMs: lysine acetylation (Kac), lysine β-hydroxybutyrylation (Kbhb), and phosphorylation. Using LC-MS/MS, we identified 4571 Kac sites, 7812 Kbhb sites, and 6237 phosphorylation sites across 2455, 3109, and 2786 proteins, respectively. Subcellular localization analysis revealed significant overlaps between Kac and Kbhb proteins, primarily in the chloroplast, cytosol, and nucleus, while phosphorylated proteins were predominantly located in the nucleus and chloroplast. Motif analysis highlighted specific amino acid enrichments around modification sites, with several motifs conserved. Additionally, 529 proteins harbored all three PTMs, underscoring the potential regulatory interplay. Kac, Kbhb, and phosphorylated proteins were particularly abundant in glycolysis, the TCA cycle, carbon fixation, and lipid metabolism pathways, influencing energy production and lipid accumulation. Based on previous transcriptome data under nutrient-limited conditions, these frequently modified key enzymes appear to be vital components in the response to abiotic stress. The presence of histone modifications related to Kac and Kbhb might also point to the epigenetic regulation in gene expression and stress adaptation. This comprehensive PTM landscape in N. oceanica provides a foundation of valuable insights into future metabolic engineering and biotechnological applications.
中文翻译:

海洋微拟球藻中蛋白质磷酸化、乙酰化和 β-羟基丁酰化的全球分析
蛋白质翻译后修饰 (PTM) 调节蛋白质功能,但在微拟球藻中仍然难以表征。本研究检查了三种 PTM:赖氨酸乙酰化 (Kac)、赖氨酸 β-羟基丁酰化 (Kbhb) 和磷酸化。使用 LC-MS/MS,我们分别在 2455、3109 和 2786 种蛋白质中鉴定了 4571 个 Kac 位点、7812 个 Kbhb 位点和 6237 个磷酸化位点。亚细胞定位分析显示 Kac 和 Kbhb 蛋白之间有显著重叠,主要在叶绿体、胞质溶胶和细胞核中,而磷酸化蛋白主要位于细胞核和叶绿体中。基序分析突出了修饰位点周围的特异性氨基酸富集,其中几个基序是保守的。此外,529 种蛋白质包含所有三种 PTM,强调了潜在的调节相互作用。Kac 、 Kbhb 和磷酸化蛋白在糖酵解、 TCA 循环、碳固定和脂质代谢途径中特别丰富,影响能量产生和脂质积累。根据先前在营养有限条件下的转录组数据,这些经常修饰的关键酶似乎是响应非生物胁迫的重要组成部分。与 Kac 和 Kbhb 相关的组蛋白修饰的存在也可能指向基因表达和应激适应中的表观遗传调控。大洋猪笼草的这种全面的 PTM 景观为未来的代谢工程和生物技术应用提供了宝贵的见解基础。
更新日期:2024-11-14
中文翻译:

海洋微拟球藻中蛋白质磷酸化、乙酰化和 β-羟基丁酰化的全球分析
蛋白质翻译后修饰 (PTM) 调节蛋白质功能,但在微拟球藻中仍然难以表征。本研究检查了三种 PTM:赖氨酸乙酰化 (Kac)、赖氨酸 β-羟基丁酰化 (Kbhb) 和磷酸化。使用 LC-MS/MS,我们分别在 2455、3109 和 2786 种蛋白质中鉴定了 4571 个 Kac 位点、7812 个 Kbhb 位点和 6237 个磷酸化位点。亚细胞定位分析显示 Kac 和 Kbhb 蛋白之间有显著重叠,主要在叶绿体、胞质溶胶和细胞核中,而磷酸化蛋白主要位于细胞核和叶绿体中。基序分析突出了修饰位点周围的特异性氨基酸富集,其中几个基序是保守的。此外,529 种蛋白质包含所有三种 PTM,强调了潜在的调节相互作用。Kac 、 Kbhb 和磷酸化蛋白在糖酵解、 TCA 循环、碳固定和脂质代谢途径中特别丰富,影响能量产生和脂质积累。根据先前在营养有限条件下的转录组数据,这些经常修饰的关键酶似乎是响应非生物胁迫的重要组成部分。与 Kac 和 Kbhb 相关的组蛋白修饰的存在也可能指向基因表达和应激适应中的表观遗传调控。大洋猪笼草的这种全面的 PTM 景观为未来的代谢工程和生物技术应用提供了宝贵的见解基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号