当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulating the electronic structure of catalysts via stable ceria and adjustable copper metal/oxide towards efficient overall water splitting
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-11-13 , DOI: 10.1039/d4ta06305h Huan Zheng, Tao Yin, Jialong Yu, Wei Xu, Weizhen Zhang, Qihui Yu, Yingnan Guo, Li Guan, Xiaolei Huang, Fenghe Wang
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-11-13 , DOI: 10.1039/d4ta06305h Huan Zheng, Tao Yin, Jialong Yu, Wei Xu, Weizhen Zhang, Qihui Yu, Yingnan Guo, Li Guan, Xiaolei Huang, Fenghe Wang
|
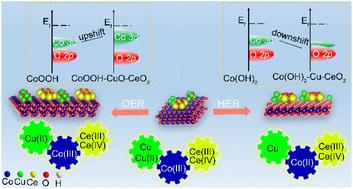
Designing efficient, economical bifunctional electrocatalysts for overall water splitting is important and challenging. This paper demonstrates a cobalt-based electrocatalyst modified with stable ceria (CeO2) and adjustable copper metal/oxide (Cu/CuO) to regulate electrochemical reconfigurations during the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Theoretical calculations reveal that CeO2 substantially impacts the electronic configuration and D-band center of catalysts. Surprisingly, CeO2 moves the D-band center of cobalt oxyhydroxide closer to the Fermi level but shifts the D-band center of cobalt hydroxide away from the Fermi level. Therefore, CeO2 optimizes the adsorption energy of the intermediates and boosts the OER activity, while reducing the ability of absorbed hydrogen atoms to bond with the catalyst and enhancing the electron-donor performance of the catalyst in the HER. Furthermore, the presence of Cu/CuO dramatically improves the catalytic activity. Hence, the utilization of CeO2 and CuO/Cu in cobalt-based nanosheet arrays enhances catalytic efficiency in overall water splitting. The overpotentials are only 94 mV and 246 mV (@10 mA cm−2) for the HER and OER, respectively, superior to those of pure cobalt-based catalysts. This study presents an innovative approach to developing efficient overall water splitting catalysts and offers insights into future developments in this field.
中文翻译:

通过稳定的铈和可调节的铜金属/氧化物调节催化剂的电子结构,以实现高效的整体水分解
为整体分解水设计高效、经济的双功能电催化剂非常重要且具有挑战性。本文展示了一种用稳定铈 (CeO2) 和可调节铜金属/氧化物 (Cu/CuO) 改性的钴基电催化剂,用于调节析氢反应 (HER) 和析氧反应 (OER) 过程中的电化学重构。理论计算表明,CeO2 对催化剂的电子构型和 D 波段中心有很大影响。令人惊讶的是,CeO2 使氢氧化钴的 D 带中心更接近费米能级,但将氢氧化钴的 D 带中心从费米能级移开。因此,CeO2 优化了中间体的吸附能并提高了 OER 活性,同时降低了吸收的氢原子与催化剂结合的能力,并增强了催化剂在 HER 中的电子供体性能。此外,Cu/CuO 的存在显着提高了催化活性。因此,在钴基纳米片阵列中利用 CeO2 和 CuO/Cu 可以提高整体水分解的催化效率。HER 和 OER 的过电位分别仅为 94 mV 和 246 mV (@10 mA cm-2),优于纯钴基催化剂的过电位。本研究提出了一种开发高效整体分解水催化剂的创新方法,并为该领域的未来发展提供了见解。
更新日期:2024-11-13
中文翻译:

通过稳定的铈和可调节的铜金属/氧化物调节催化剂的电子结构,以实现高效的整体水分解
为整体分解水设计高效、经济的双功能电催化剂非常重要且具有挑战性。本文展示了一种用稳定铈 (CeO2) 和可调节铜金属/氧化物 (Cu/CuO) 改性的钴基电催化剂,用于调节析氢反应 (HER) 和析氧反应 (OER) 过程中的电化学重构。理论计算表明,CeO2 对催化剂的电子构型和 D 波段中心有很大影响。令人惊讶的是,CeO2 使氢氧化钴的 D 带中心更接近费米能级,但将氢氧化钴的 D 带中心从费米能级移开。因此,CeO2 优化了中间体的吸附能并提高了 OER 活性,同时降低了吸收的氢原子与催化剂结合的能力,并增强了催化剂在 HER 中的电子供体性能。此外,Cu/CuO 的存在显着提高了催化活性。因此,在钴基纳米片阵列中利用 CeO2 和 CuO/Cu 可以提高整体水分解的催化效率。HER 和 OER 的过电位分别仅为 94 mV 和 246 mV (@10 mA cm-2),优于纯钴基催化剂的过电位。本研究提出了一种开发高效整体分解水催化剂的创新方法,并为该领域的未来发展提供了见解。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号