当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative Coupling of Primary Benzamide with Alkenes via o-CH Activation Mediated by Cu(II)/Ru(II)
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.joc.4c00637 Aditi Soni, Lalit Negi, Charu Sharma, Avinash K. Srivastava, Raj K. Joshi
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.joc.4c00637 Aditi Soni, Lalit Negi, Charu Sharma, Avinash K. Srivastava, Raj K. Joshi
|
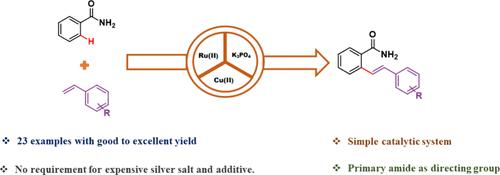
A selective ortho-olefination of primary amides yields valuable motifs for pharmaceuticals. Primary amide-directed ortho-olefination of benzamide using unactivated alkenes was successfully conducted with a ruthenium(II) catalyst. The established protocol demonstrates efficacy across various benzamides, achieving moderate to good yields with high functional group tolerance. Furthermore, the transformation of ortho-olefinated amides into five-membered lactams highlights their synthetic applicability in the pharmaceutical sector.
中文翻译:

伯苯甲酰胺与烯烃通过 Cu(II)/Ru(II) 介导的 o-CH 活化的氧化偶联
伯酰胺的选择性邻烯化可产生有价值的药物基序。使用未活化烯烃对苯甲酰胺进行伯酰胺定向邻烯烃反应,使用钌 (II) 催化剂成功进行。既定方案在各种苯甲酰胺中均有效,可实现中等至良好的产量和高官能团耐受性。此外,邻位烯烃酰胺转化为五元内酰胺突出了它们在制药领域的合成适用性。
更新日期:2024-11-13
中文翻译:

伯苯甲酰胺与烯烃通过 Cu(II)/Ru(II) 介导的 o-CH 活化的氧化偶联
伯酰胺的选择性邻烯化可产生有价值的药物基序。使用未活化烯烃对苯甲酰胺进行伯酰胺定向邻烯烃反应,使用钌 (II) 催化剂成功进行。既定方案在各种苯甲酰胺中均有效,可实现中等至良好的产量和高官能团耐受性。此外,邻位烯烃酰胺转化为五元内酰胺突出了它们在制药领域的合成适用性。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号