当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of Spiro[pyrrolidine-azlactone] via [2 + 3] Cycloaddition of Alkylidene Azlactone with Trifluoromethylated Imine
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.joc.4c02093 Lesong Li, Xia Wang, Yang Wang
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.joc.4c02093 Lesong Li, Xia Wang, Yang Wang
|
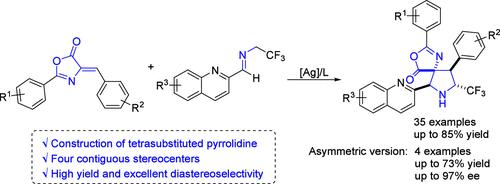
A Ag-catalyzed [2 + 3] cycloaddition of alkylidene azlactones with trifluoromethylated imines has been documented, providing spiro[pyrrolidine-azlactones] bearing four adjacent stereogenic centers with good yields and excellent diastereoselectivities. Catalytic asymmetric cycloaddition has also been developed with good yields and excellent enantioselectivities. Further gram-scale preparation and synthetic transformation to pyrrolidine derivative showed the good practicality and applicability of this reaction.
中文翻译:

通过烷基氮化氮内酯与三氟甲基化亚胺的 [2 + 3] 环加成反应构建螺 [吡咯烷-氮内酯]
已经记录了烷基氮杂内酯与三氟甲基化亚胺的 Ag 催化 [2 + 3] 环加成反应,提供带有四个相邻立体中心的螺体 [吡咯烷-氮唑酮] 具有良好的产率和优异的非对映选择性。催化不对称环加成反应也已被开发出来,具有良好的产率和优异的对映选择性。进一步的克级制备和合成转化为吡咯烷衍生物表明该反应具有良好的实用性和适用性。
更新日期:2024-11-13
中文翻译:

通过烷基氮化氮内酯与三氟甲基化亚胺的 [2 + 3] 环加成反应构建螺 [吡咯烷-氮内酯]
已经记录了烷基氮杂内酯与三氟甲基化亚胺的 Ag 催化 [2 + 3] 环加成反应,提供带有四个相邻立体中心的螺体 [吡咯烷-氮唑酮] 具有良好的产率和优异的非对映选择性。催化不对称环加成反应也已被开发出来,具有良好的产率和优异的对映选择性。进一步的克级制备和合成转化为吡咯烷衍生物表明该反应具有良好的实用性和适用性。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号