当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Correction to “Synthesis of a 3′-Fluoro-3′-deoxytetrose Adenine Phosphonate”
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.joc.4c02215 Swarup De, Steven De Jonghe, Piet Herdewijn
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.joc.4c02215 Swarup De, Steven De Jonghe, Piet Herdewijn
|
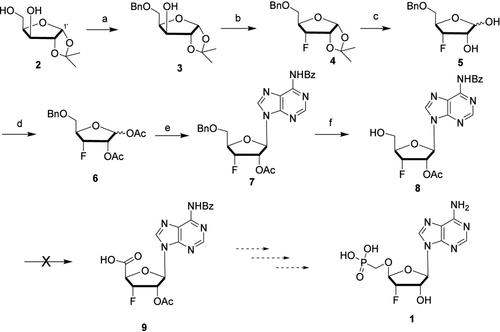
The assignment of the structure of compound 3 in Scheme 1 was incorrect (presented here as Figure 1). A reported literature procedure (Org. Lett. 2000, 2, 3355–3357), which is also referenced in the manuscript (ref 13), was followed to synthesize compound 3. However, in the same paper, no NMR spectra were provided, and for structural identification, the authors referred to Wong et al. (J. Am. Chem. Soc. 1998, 120, 1965–1978). After comparing all of the NMR data of compound 3 with previously reported data, we came to the conclusion that the isolated compound was primarily 3′-benzylated adduct 3a (Figure 2) with only a minor presence of the desired compound 3. On the basis of the assumption that we made the correct regioisomer of 3 (i.e., the 5′-benzylated analogue), the following steps were performed. We now show the correct structures of compounds 3–8 in Figure 2 that were isolated in the following steps. Figure 1. Scheme 1 from the original work. Figure 2. Revised Scheme 1 with the correct structures. We think that the details of compounds 4a–8a in Scheme 1 of the original paper are now redundant. Therefore, we remove this information from the original paper and its Supporting Information. The other parts of the original paper remain unchanged, and there is no consequence of the unintentional misinterpretation in the conclusion. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.4c02215. 1H, 13C, 19F, and 31P NMR spectra and HRMS spectra of intermediates and final compounds (PDF) Correction to “Synthesis
of a 3′-Fluoro-3′-deoxytetrose
Adenine Phosphonate” 0 views 0 shares 0 downloads Most electronic Supporting Information files are available without a subscription to ACS Web Editions. Such files may be downloaded by article for research use (if there is a public use license linked to the relevant article, that license may permit other uses). Permission may be obtained from ACS for other uses through requests via the RightsLink permission system: http://pubs.acs.org/page/copyright/permissions.html. This article has not yet been cited by other publications.
中文翻译:

更正为“3′-氟-3′-脱氧四氟化物腺嘌呤膦酸盐的合成”
方案 1 中化合物 3 的结构分配不正确(如图 1 所示)。一个已报道的文献程序 (Org. Lett.2000, 2, 3355–3357),该化合物在手稿(参考文献 13)中也被引用,用于合成化合物 3。然而,在同一篇论文中,没有提供 NMR 波谱,对于结构鉴定,作者引用了 Wong 等人 (J. Am. Chem. Soc.1998, 120, 1965–1978)。在将化合物 3 的所有 NMR 数据与之前报告的数据进行比较后,我们得出结论,分离的化合物主要是 3′-苄基化加合物 3a(图 2),只有少量所需的化合物 3。基于我们制备了正确的区域异构体 3(即 5′-苄基化类似物)的基础,执行以下步骤。现在,我们展示了图 2 中化合物 3-8 的正确结构,这些化合物在以下步骤中分离出来。图 1.方案 1 来自原始作品。图 2.修改了具有正确结构的方案 1。我们认为原始论文方案 1 中化合物 4a-8a 的细节现在是多余的。因此,我们从原始论文及其支持信息中删除此信息。原文的其他部分保持不变,结论中没有无意曲解的后果。支持信息可在 https://pubs.acs.org/doi/10.1021/acs.joc.4c02215 免费获取。 1中间体和最终化合物的 H、13C、19F 和 31P NMR 波谱和 HRMS 波谱 (PDF) 更正为“3′-氟-3′-脱氧赤膠糖的合成” 0 次浏览 0 分享 0 下载 大多数电子支持信息文件无需订阅 ACS Web 版即可获得。此类文件可以按文章下载用于研究用途(如果有链接到相关文章的公共使用许可证,则该许可证可能允许其他用途)。可以通过 RightsLink 权限系统(http://pubs.acs.org/page/copyright/permissions.html)请求,从 ACS 获得用于其他用途的权限。本文尚未被其他出版物引用。
更新日期:2024-11-13
中文翻译:

更正为“3′-氟-3′-脱氧四氟化物腺嘌呤膦酸盐的合成”
方案 1 中化合物 3 的结构分配不正确(如图 1 所示)。一个已报道的文献程序 (Org. Lett.2000, 2, 3355–3357),该化合物在手稿(参考文献 13)中也被引用,用于合成化合物 3。然而,在同一篇论文中,没有提供 NMR 波谱,对于结构鉴定,作者引用了 Wong 等人 (J. Am. Chem. Soc.1998, 120, 1965–1978)。在将化合物 3 的所有 NMR 数据与之前报告的数据进行比较后,我们得出结论,分离的化合物主要是 3′-苄基化加合物 3a(图 2),只有少量所需的化合物 3。基于我们制备了正确的区域异构体 3(即 5′-苄基化类似物)的基础,执行以下步骤。现在,我们展示了图 2 中化合物 3-8 的正确结构,这些化合物在以下步骤中分离出来。图 1.方案 1 来自原始作品。图 2.修改了具有正确结构的方案 1。我们认为原始论文方案 1 中化合物 4a-8a 的细节现在是多余的。因此,我们从原始论文及其支持信息中删除此信息。原文的其他部分保持不变,结论中没有无意曲解的后果。支持信息可在 https://pubs.acs.org/doi/10.1021/acs.joc.4c02215 免费获取。 1中间体和最终化合物的 H、13C、19F 和 31P NMR 波谱和 HRMS 波谱 (PDF) 更正为“3′-氟-3′-脱氧赤膠糖的合成” 0 次浏览 0 分享 0 下载 大多数电子支持信息文件无需订阅 ACS Web 版即可获得。此类文件可以按文章下载用于研究用途(如果有链接到相关文章的公共使用许可证,则该许可证可能允许其他用途)。可以通过 RightsLink 权限系统(http://pubs.acs.org/page/copyright/permissions.html)请求,从 ACS 获得用于其他用途的权限。本文尚未被其他出版物引用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号