当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
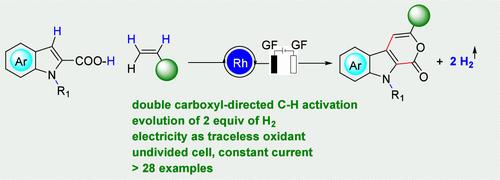
Rhodaelectro-Catalyzed Synthesis of Pyrano[3,4-b]indol-1(9H)-ones via the Double Dehydrogenative Heck Reaction between Indole-2-carboxylic Acids and Alkenes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.joc.4c02271 Fengyi Xiao, Xinlu Xu, Jiaqi Zhang, Ximan Chen, Xin Ruan, Qi Wei, Xiaofeng Zhang, Qiufeng Huang
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.joc.4c02271 Fengyi Xiao, Xinlu Xu, Jiaqi Zhang, Ximan Chen, Xin Ruan, Qi Wei, Xiaofeng Zhang, Qiufeng Huang
|
A rhodaelectro-catalyzed double dehydrogenative Heck reaction of indole-2-carboxylic acids with alkenes has been developed for the synthesis of pyrano[3,4-b]indol-1(9H)-ones. The weakly coordinating carboxyl group is utilized twice as a directing group to activate the C–H bonds throughout the reaction. This reaction precedes an acceptorless dehydrogenation under exogenous oxidant-free conditions in an undivided cell with a constant current.
中文翻译:

通过吲哚-2-羧酸和烯烃之间的双重脱氢 Heck 反应,罗达电催化合成吡哒[3,4-b]吲哚-1(9H)-酮
已经开发了一种吲哚-2-羧酸与烯烃的电催化双脱氢 Heck 反应,用于合成吡喃[3,4-b]吲哚-1(9H)-酮。弱配位的羧基被用作定向基团两次,以在整个反应过程中激活 C-H 键。该反应在无外源性氧化剂条件下,在恒定电流的未分流池中进行无受体脱氢之前。
更新日期:2024-11-13
中文翻译:

通过吲哚-2-羧酸和烯烃之间的双重脱氢 Heck 反应,罗达电催化合成吡哒[3,4-b]吲哚-1(9H)-酮
已经开发了一种吲哚-2-羧酸与烯烃的电催化双脱氢 Heck 反应,用于合成吡喃[3,4-b]吲哚-1(9H)-酮。弱配位的羧基被用作定向基团两次,以在整个反应过程中激活 C-H 键。该反应在无外源性氧化剂条件下,在恒定电流的未分流池中进行无受体脱氢之前。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号