当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polysubstituted Pyridines from 1,4-Oxazinone Precursors
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.joc.4c02389 L. C. Thompson, Adrianne M. Kinsey, Zannatul Shahla, Jonathan R. Scheerer
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.joc.4c02389 L. C. Thompson, Adrianne M. Kinsey, Zannatul Shahla, Jonathan R. Scheerer
|
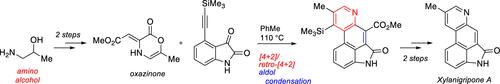
This study describes a general method for the preparation of 1,4-oxazin-2-one intermediates from acetylene dicarboxylate and β-amino alcohol precursors. Oxazinones prepared in this manner were employed in a tandem cycloaddition/cycloreversion reaction sequence with a model alkyne (phenyl acetylene) to give substituted pyridine products. Fundamental reactivity and selectivity studies are complemented by the synthesis of the polycyclic ergot alkaloid natural product xylanigripone A.
中文翻译:

来自 1,4-恶嗪酮前体的多取代吡啶
本研究描述了一种从乙炔二羧酸盐和 β-氨基醇前体制备 1,4-恶嗪-2-酮中间体的通用方法。以这种方式制备的恶嗪酮与模型炔烃(苯基乙炔)在串联环加成/环反转反应序列中用于得到取代的吡啶产物。基本反应性和选择性研究由多环麦角生物碱天然产物二基苯醚 A 的合成补充。
更新日期:2024-11-13
中文翻译:

来自 1,4-恶嗪酮前体的多取代吡啶
本研究描述了一种从乙炔二羧酸盐和 β-氨基醇前体制备 1,4-恶嗪-2-酮中间体的通用方法。以这种方式制备的恶嗪酮与模型炔烃(苯基乙炔)在串联环加成/环反转反应序列中用于得到取代的吡啶产物。基本反应性和选择性研究由多环麦角生物碱天然产物二基苯醚 A 的合成补充。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号