当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Regular Ladder-Type Fluorenylidene-Xanthene Oligomers via Nucleophilic Aromatic Substitution Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.orglett.4c03779 Mengmeng Gao, Xiangxuan Cui, Luna Yang, Hongchi Zhao, Yonggang Wu
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.orglett.4c03779 Mengmeng Gao, Xiangxuan Cui, Luna Yang, Hongchi Zhao, Yonggang Wu
|
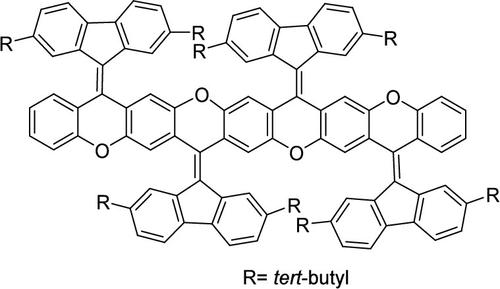
Fluorenylidene-xanthene and related polycyclic systems are typical examples of bistricyclic aromatic enes (BAEs); however, polycyclic systems of fluorenylidene-xanthene are relatively scarce. Here we synthesized a series of novel ladder-type polycyclic systems containing fluorenylidene-xanthene units, differing from the classic Barton’s two-fold extrusion reaction. In the new synthetic scheme, the very popular Suzuki reaction was first used to couple the corresponding precursors, then a catalyst-free nucleophilic aromatic substitution reaction between Ar–F and Ar–OH groups in the backbone afforded ladder-type O-heteroarenes. Moreover, the new synthetic strategy is also suitable for the synthesis of other heterocyclic and polycyclic aromatic hydrocarbons. Some of these products showed reversible mechanochromism properties.
中文翻译:

通过亲核芳香族取代反应合成规则梯形芴基-氧杂蒽低聚物
芴基-氧杂蒽和相关的多环体系是双三环芳香烯 (BAE) 的典型例子;然而,芴基-氧杂蒽的多环系统相对稀缺。在这里,我们合成了一系列包含芴基-氧杂蒽单元的新型梯形多环系统,与经典的 Barton 二重挤压反应不同。在新的合成方案中,非常流行的 Suzuki 反应首先用于偶联相应的前驱体,然后在主链中的 Ar-F 和 Ar-OH 基团之间进行无催化剂的亲核芳香族取代反应,得到梯形 O-杂芳烃。此外,新的合成策略也适用于其他杂环和多环芳烃的合成。其中一些产品显示出可逆的机械变色特性。
更新日期:2024-11-13
中文翻译:

通过亲核芳香族取代反应合成规则梯形芴基-氧杂蒽低聚物
芴基-氧杂蒽和相关的多环体系是双三环芳香烯 (BAE) 的典型例子;然而,芴基-氧杂蒽的多环系统相对稀缺。在这里,我们合成了一系列包含芴基-氧杂蒽单元的新型梯形多环系统,与经典的 Barton 二重挤压反应不同。在新的合成方案中,非常流行的 Suzuki 反应首先用于偶联相应的前驱体,然后在主链中的 Ar-F 和 Ar-OH 基团之间进行无催化剂的亲核芳香族取代反应,得到梯形 O-杂芳烃。此外,新的合成策略也适用于其他杂环和多环芳烃的合成。其中一些产品显示出可逆的机械变色特性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号