Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The ALS drug riluzole binds to the C-terminal domain of SARS-CoV-2 nucleocapsid protein and has antiviral activity
Structure ( IF 4.4 ) Pub Date : 2024-11-13 , DOI: 10.1016/j.str.2024.10.025 María Ángeles Márquez-Moñino, Clara M. Santiveri, Patricia de León, Sergio Camero, Ramón Campos-Olivas, M. Ángeles Jiménez, Margarita Sáiz, Beatriz González, José Manuel Pérez-Cañadillas
Structure ( IF 4.4 ) Pub Date : 2024-11-13 , DOI: 10.1016/j.str.2024.10.025 María Ángeles Márquez-Moñino, Clara M. Santiveri, Patricia de León, Sergio Camero, Ramón Campos-Olivas, M. Ángeles Jiménez, Margarita Sáiz, Beatriz González, José Manuel Pérez-Cañadillas
|
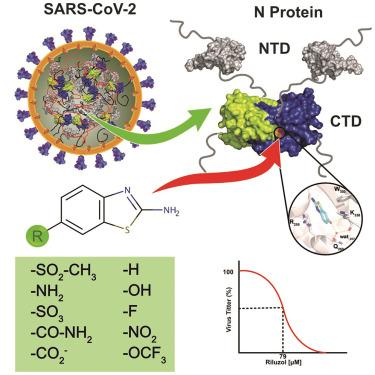
Nucleoproteins (N) play an essential role in virus assembly and are less prone to mutation than other viral structural proteins, making them attractive targets for drug discovery. Using an NMR fragment-based drug discovery approach, we identified the 1,3-benzothiazol-2-amine (BZT) group as a scaffold to develop potential antivirals for SARS-CoV-2 nucleocapsid (N) protein. A thorough characterization of BZT derivatives using NMR, X-ray crystallography, antiviral activity assays, and intrinsic fluorescence measurements revealed their binding in the C-terminal domain (CTD) domain of the N protein, to residues Arg 259, Trp 330, and Lys 338, coinciding with the nucleotide binding site. Our most effective compound exhibits a slightly better affinity than GTP and the ALS drug riluzole, also identified during the screening, and displays notable viral inhibition activity. A virtual screening of 218 BZT-based compounds revealed a potential extended binding site that could be exploited for the future development of new SARS-CoV-2 antivirals.
中文翻译:

ALS 药物利鲁唑与 SARS-CoV-2 核衣壳蛋白的 C 端结构域结合,具有抗病毒活性
核蛋白 (N) 在病毒组装中起着至关重要的作用,与其他病毒结构蛋白相比,不易发生突变,使其成为药物发现的有吸引力的靶标。使用基于 NMR 片段的药物发现方法,我们确定了 1,3-苯并噻唑-2-胺 (BZT) 基团作为开发 SARS-CoV-2 核衣壳 (N) 蛋白潜在抗病毒药物的支架。使用 NMR、X 射线晶体学、抗病毒活性测定和内源荧光测量对 BZT 衍生物进行全面表征,发现它们在 N 蛋白的 C 端结构域 (CTD) 结构域中与残基 Arg 259、Trp 330 和 Lys 338 结合,与核苷酸结合位点重合。我们最有效的化合物表现出比 GTP 和 ALS 药物利鲁唑略好的亲和力,后者也在筛选过程中被发现,并显示出显着的病毒抑制活性。对 218 种基于 BZT 的化合物的虚拟筛选揭示了一个潜在的扩展结合位点,可用于未来开发新的 SARS-CoV-2 抗病毒药物。
更新日期:2024-11-13
中文翻译:

ALS 药物利鲁唑与 SARS-CoV-2 核衣壳蛋白的 C 端结构域结合,具有抗病毒活性
核蛋白 (N) 在病毒组装中起着至关重要的作用,与其他病毒结构蛋白相比,不易发生突变,使其成为药物发现的有吸引力的靶标。使用基于 NMR 片段的药物发现方法,我们确定了 1,3-苯并噻唑-2-胺 (BZT) 基团作为开发 SARS-CoV-2 核衣壳 (N) 蛋白潜在抗病毒药物的支架。使用 NMR、X 射线晶体学、抗病毒活性测定和内源荧光测量对 BZT 衍生物进行全面表征,发现它们在 N 蛋白的 C 端结构域 (CTD) 结构域中与残基 Arg 259、Trp 330 和 Lys 338 结合,与核苷酸结合位点重合。我们最有效的化合物表现出比 GTP 和 ALS 药物利鲁唑略好的亲和力,后者也在筛选过程中被发现,并显示出显着的病毒抑制活性。对 218 种基于 BZT 的化合物的虚拟筛选揭示了一个潜在的扩展结合位点,可用于未来开发新的 SARS-CoV-2 抗病毒药物。































 京公网安备 11010802027423号
京公网安备 11010802027423号