Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unusually high selectivity (100 %) of photocatalytic C[sbnd]C coupling achieved by instant reverse reduction of byproducts
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-12 , DOI: 10.1016/j.jcat.2024.115842 Tianhan Shen, Qipeng Chen, Yue Gao, Zuofei Gu, Huiqing Zhang, Fengyan Shi, Guohua Liu, Yuning Huo, Hexing Li
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-12 , DOI: 10.1016/j.jcat.2024.115842 Tianhan Shen, Qipeng Chen, Yue Gao, Zuofei Gu, Huiqing Zhang, Fengyan Shi, Guohua Liu, Yuning Huo, Hexing Li
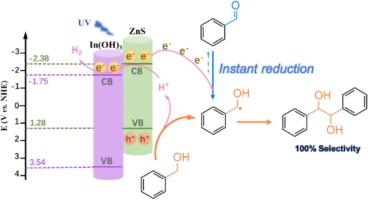
|
The photocatalytic C3 -ZnS photocatalyst for C3 onto ZnS with stable interaction facilitates light harvesting and separation of photo-excited charges. As a result, BA conversion on optimized In(0.1)-ZnS catalyst (73 %) is much higher than ZnS (29 %). Besides, the surface hydroxyl groups derived from In(OH)3 enables the facile desorption of
中文翻译:

通过即时反向还原副产物实现光催化 C[sbnd]C 偶联的异常高选择性 (100%)
苯甲醇 (BA) 与氢安息香 (HB) 的光催化 CC 偶联,对于获得高价值化学品具有吸引力。然而,由于不可避免地会形成苯甲醛,HB 的选择性仍然很低。在此,我们报道了 In(OH)3-ZnS 光催化剂,用于 BA 与 HB 的 CC 偶联,具有非常高的选择性 (∼100 %)。将 In(OH)3 引入 ZnS 上并具有稳定的相互作用,有助于光的收集和光激发电荷的分离。因此,优化的 In(0.1)-ZnS 催化剂的 BA 转化率 (73 %) 远高于 ZnS (29 %)。此外,In(OH)3 衍生的表面羟基能够轻松解吸 CH(OH)Ph 自由基。因此,可以有效抑制 CH(OH)Ph 自由基过度氧化成苯甲醛副产物。更重要的是,原位 FTIR 光谱和副产物的还原表明,在 BA 的 CC 偶联过程中,苯甲醛即时逆还原为 CH(OH)Ph 自由基,这是实现满意的 HB 选择性 (100%) 的关键。理论模拟表明,CH(OH)Ph 自由基对催化剂的弱吸附和 CH(OH)Ph 过度氧化成苯甲醛的高能垒有助于形成高选择性偶联产物。这项工作将激发新的见解,以设计合理的光氧化还原系统,以实现具有高选择性的有机转化。
更新日期:2024-11-12
中文翻译:

通过即时反向还原副产物实现光催化 C[sbnd]C 偶联的异常高选择性 (100%)
苯甲醇 (BA) 与氢安息香 (HB) 的光催化 CC 偶联,对于获得高价值化学品具有吸引力。然而,由于不可避免地会形成苯甲醛,HB 的选择性仍然很低。在此,我们报道了 In(OH)3-ZnS 光催化剂,用于 BA 与 HB 的 CC 偶联,具有非常高的选择性 (∼100 %)。将 In(OH)3 引入 ZnS 上并具有稳定的相互作用,有助于光的收集和光激发电荷的分离。因此,优化的 In(0.1)-ZnS 催化剂的 BA 转化率 (73 %) 远高于 ZnS (29 %)。此外,In(OH)3 衍生的表面羟基能够轻松解吸 CH(OH)Ph 自由基。因此,可以有效抑制 CH(OH)Ph 自由基过度氧化成苯甲醛副产物。更重要的是,原位 FTIR 光谱和副产物的还原表明,在 BA 的 CC 偶联过程中,苯甲醛即时逆还原为 CH(OH)Ph 自由基,这是实现满意的 HB 选择性 (100%) 的关键。理论模拟表明,CH(OH)Ph 自由基对催化剂的弱吸附和 CH(OH)Ph 过度氧化成苯甲醛的高能垒有助于形成高选择性偶联产物。这项工作将激发新的见解,以设计合理的光氧化还原系统,以实现具有高选择性的有机转化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号