当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and biological evaluation of novel pleuromutilin derivatives with methicillin-resistant Staphylococcus aureus -targeting phenol linker groups
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-13 , DOI: 10.1016/j.ejmech.2024.117061 Yunpeng Yi, Jiaming Zhang, Shuqian Lin, Haiting Wang, Guiyu Li, Shifa Yang, Ruofeng Shang, Rongling Zhang, Fei Li
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-13 , DOI: 10.1016/j.ejmech.2024.117061 Yunpeng Yi, Jiaming Zhang, Shuqian Lin, Haiting Wang, Guiyu Li, Shifa Yang, Ruofeng Shang, Rongling Zhang, Fei Li
|
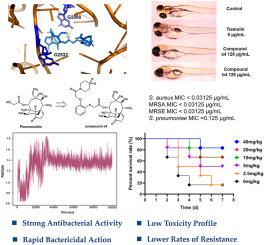
Methicillin-resistant Staphylococcus aureus (MRSA) remains a significant global health threat, necessitating the development of new therapeutic agents. Pleuromutilin derivatives offer a promising solution due to their potent antibacterial activity, particularly against Gram-positive bacteria such as MRSA. In this study, we synthesized a series of pleuromutilin derivatives with phenol linker containing C14 side chains and evaluated in vitro and in vivo antibacterial activities. Several compounds showed potent activity against MRSA and Staphylococcus aureus with minimal inhibitory concentrations (MICs) as low as 0.03125 μg/mL. In particular, compounds a4 and b4 showed rapid bactericidal activity, significantly reducing MRSA loads in time-kill kinetics and exhibiting slower resistance development compared to tiamulin. In vivo , compound a4 showed superior efficacy in reducing MRSA-induced lung damage in a mouse model at a lower effective dose (ED50 = 6.48 mg/kg) compared to tiamulin (ED50 = 11.38 mg/kg). Molecular docking and molecular dynamics studies also showed that compound a4 binds strongly to the ribosomal peptidyl transferase center (PTC), a key target for pleuromutilin derivatives. These results suggest that compound a4 , with its enhanced antibacterial activity and low resistance potential, is a promising candidate for further development as an effective treatment for MRSA infections.
中文翻译:

耐甲氧西林金黄色葡萄球菌靶向苯酚接头基团的新型倍膜MUTILIN衍生物的设计、合成和生物学评价
耐甲氧西林金黄色葡萄球菌 (MRSA) 仍然是一个重大的全球健康威胁,需要开发新的治疗药物。Pleuromutilin 衍生物由于其强大的抗菌活性,特别是对 MRSA 等革兰氏阳性菌,提供了一种有前途的解决方案。在这项研究中,我们用含有 C14 侧链的苯酚接头合成了一系列 pleuromutilin 衍生物,并评价了体外和体内的抗菌活性。几种化合物对 MRSA 和金黄色葡萄球菌显示出强效活性,最低抑菌浓度 (MIC) 低至 0.03125 μg/mL。特别是,化合物 a4 和 b4 显示出快速的杀菌活性,显著降低了 MRSA 在时间杀灭动力学中的负荷,并且与 tiamulin 相比表现出较慢的耐药性发展。在体内,与tiamulin (ED50 = 11.38 mg/kg) 相比,化合物 a4 在小鼠模型中以较低的有效剂量 (ED50 = 6.48 mg/kg) 在减少 MRSA 诱导的肺损伤方面显示出卓越的疗效。分子对接和分子动力学研究还表明,化合物 a4 与核糖体肽基转移酶中心 (PTC) 强烈结合,PTC 是 pleuromutilin 衍生物的关键靶标。这些结果表明,化合物 a4 具有增强的抗菌活性和低耐药性潜力,是进一步开发作为 MRSA 感染有效治疗方法的有前途的候选者。
更新日期:2024-11-13
中文翻译:

耐甲氧西林金黄色葡萄球菌靶向苯酚接头基团的新型倍膜MUTILIN衍生物的设计、合成和生物学评价
耐甲氧西林金黄色葡萄球菌 (MRSA) 仍然是一个重大的全球健康威胁,需要开发新的治疗药物。Pleuromutilin 衍生物由于其强大的抗菌活性,特别是对 MRSA 等革兰氏阳性菌,提供了一种有前途的解决方案。在这项研究中,我们用含有 C14 侧链的苯酚接头合成了一系列 pleuromutilin 衍生物,并评价了体外和体内的抗菌活性。几种化合物对 MRSA 和金黄色葡萄球菌显示出强效活性,最低抑菌浓度 (MIC) 低至 0.03125 μg/mL。特别是,化合物 a4 和 b4 显示出快速的杀菌活性,显著降低了 MRSA 在时间杀灭动力学中的负荷,并且与 tiamulin 相比表现出较慢的耐药性发展。在体内,与tiamulin (ED50 = 11.38 mg/kg) 相比,化合物 a4 在小鼠模型中以较低的有效剂量 (ED50 = 6.48 mg/kg) 在减少 MRSA 诱导的肺损伤方面显示出卓越的疗效。分子对接和分子动力学研究还表明,化合物 a4 与核糖体肽基转移酶中心 (PTC) 强烈结合,PTC 是 pleuromutilin 衍生物的关键靶标。这些结果表明,化合物 a4 具有增强的抗菌活性和低耐药性潜力,是进一步开发作为 MRSA 感染有效治疗方法的有前途的候选者。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号