当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural insights into tryptamine psychedelics: The role of hydroxyl indole ring site in 5-HT2A receptor activation and psychedelic-like activity
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-12 , DOI: 10.1016/j.ejmech.2024.117049 Miyuan Zhang, Yuefeng Yang, Zhishuai Yang, Xin Wen, Cong Zhang, Peng Xiao, Yibo Wang, Jinpeng Sun, Hongshuang Wang, Xiaohui Wang
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-12 , DOI: 10.1016/j.ejmech.2024.117049 Miyuan Zhang, Yuefeng Yang, Zhishuai Yang, Xin Wen, Cong Zhang, Peng Xiao, Yibo Wang, Jinpeng Sun, Hongshuang Wang, Xiaohui Wang
|
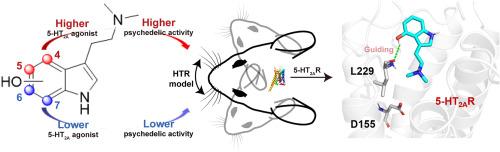
Recent advancements in the study of mushroom-derived tryptamines, particularly psilocybin and its metabolite psilocin, highlight their unique psychedelic properties and potential therapeutic applications, especially for mental health conditions like depression. This study examines how the position of the hydroxyl group on the indole ring affects the 5-HT2A receptor activity and psychedelic-like effects of psilocin analogs. Chemically synthesized psilocin (1 ) and its analogs bufotenine (2 ), 6-OH-DMT (3 ), and 7-OH-DMT (4 ) were assessed for 5-HT2A receptor agonistic activity using the Gαq -Gγ dissociation bioluminescence resonance energy transfer (BRET) assay and for psychedelic-like effects through the head-twitch response assay. Results show that compounds with hydroxyl group at the 4th and 5th positions exhibit significantly higher 5-HT2A agonistic and psychedelic-like activities than those with hydroxyl group at the 6th and 7th positions. Funnel metadynamics simulations revealed that psilocin (1 ) and bufotenine (2 ) have lower binding free energies, correlating with experimental data. Analysis of the simulation trajectories reveals that the formation of a hydrogen bond with residue L229 is crucial for guiding psilocin (1 ) and bufotenine (2 ) into the 5-HT2A R binding site. In contrast, analogs 3 and 4 , which lack this interaction, fail to be directed into the orthosteric site. Furthermore, psilocin (1 ) and bufotenine (2 ) establish a stable salt bridge and hydrogen bond with residue D155. These interactions are more stable compared to those formed by ligands 3 and 4 , contributing to the latter's poor 5-HT2A R activities. These findings underscore the critical role of the hydroxyl group position on the indole ring in modulating 5-HT2A receptor activity and the corresponding psychedelic-like effects, offering valuable insights for the development of targeted therapeutics.
中文翻译:

色胺迷幻药的结构见解:羟基吲哚环位点在 5-HT2A 受体激活和迷幻药样活性中的作用
蘑菇衍生色胺,特别是裸盖菇素及其代谢物裸盖菇素的研究的最新进展,突出了它们独特的迷幻特性和潜在的治疗应用,特别是对于抑郁症等心理健康状况。本研究研究了吲哚环上羟基的位置如何影响 5-HT2A 受体活性和裸盖菇素类似物的迷幻作用。使用 Gαq-Gγ 解离生物发光共振能量转移 (BRET) 测定评估化学合成的裸盖菇素 (1) 及其类似物布福素 (2)、6-OH-DMT (3) 和 7-OH-DMT (4) 的 5-HT2A 受体激动活性,并通过头部抽搐反应测定评估迷幻样效果。结果表明,第 4 位和第 5 位具有羟基的化合物表现出显著高于第 6 位和第 7 位羟基的化合物的 5-HT2A 激动和迷幻样活性。漏斗元动力学模拟显示,裸盖菇素 (1) 和丁磷烯碱 (2) 具有较低的结合自由能,这与实验数据相关。模拟轨迹分析表明,与残基 L229 形成氢键对于引导裸盖菇素 (1) 和布夫欣 (2) 进入 5-HT2AR 结合位点至关重要。相比之下,缺乏这种交互的类似物 3 和 4 无法被引导到正构位点。此外,裸盖菇素 (1) 和丁磷烯碱 (2) 与残基 D155 建立稳定的盐桥和氢键。与配体 3 和 4 形成的相互作用相比,这些相互作用更稳定,导致后者的 5-HT2AR 活性较差。 这些发现强调了吲哚环上的羟基位置在调节 5-HT2A 受体活性和相应的迷幻药样作用中的关键作用,为靶向疗法的开发提供了有价值的见解。
更新日期:2024-11-12
中文翻译:

色胺迷幻药的结构见解:羟基吲哚环位点在 5-HT2A 受体激活和迷幻药样活性中的作用
蘑菇衍生色胺,特别是裸盖菇素及其代谢物裸盖菇素的研究的最新进展,突出了它们独特的迷幻特性和潜在的治疗应用,特别是对于抑郁症等心理健康状况。本研究研究了吲哚环上羟基的位置如何影响 5-HT2A 受体活性和裸盖菇素类似物的迷幻作用。使用 Gαq-Gγ 解离生物发光共振能量转移 (BRET) 测定评估化学合成的裸盖菇素 (1) 及其类似物布福素 (2)、6-OH-DMT (3) 和 7-OH-DMT (4) 的 5-HT2A 受体激动活性,并通过头部抽搐反应测定评估迷幻样效果。结果表明,第 4 位和第 5 位具有羟基的化合物表现出显著高于第 6 位和第 7 位羟基的化合物的 5-HT2A 激动和迷幻样活性。漏斗元动力学模拟显示,裸盖菇素 (1) 和丁磷烯碱 (2) 具有较低的结合自由能,这与实验数据相关。模拟轨迹分析表明,与残基 L229 形成氢键对于引导裸盖菇素 (1) 和布夫欣 (2) 进入 5-HT2AR 结合位点至关重要。相比之下,缺乏这种交互的类似物 3 和 4 无法被引导到正构位点。此外,裸盖菇素 (1) 和丁磷烯碱 (2) 与残基 D155 建立稳定的盐桥和氢键。与配体 3 和 4 形成的相互作用相比,这些相互作用更稳定,导致后者的 5-HT2AR 活性较差。 这些发现强调了吲哚环上的羟基位置在调节 5-HT2A 受体活性和相应的迷幻药样作用中的关键作用,为靶向疗法的开发提供了有价值的见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号