当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiomeric C-6 fluorinated swainsonine derivatives as highly selective and potent inhibitors of α-mannosidase and α-l-rhamnosidase: Design, synthesis and structure-activity relationship study
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-12 , DOI: 10.1016/j.ejmech.2024.117031 Feng-Teng Gao, Ming Zhang, Yuna Shimadate, Atsushi Kato, Yi-Xian Li, Yue-Mei Jia, Chu-Yi Yu
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-12 , DOI: 10.1016/j.ejmech.2024.117031 Feng-Teng Gao, Ming Zhang, Yuna Shimadate, Atsushi Kato, Yi-Xian Li, Yue-Mei Jia, Chu-Yi Yu
|
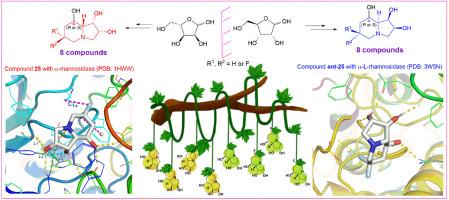
Six C-6 fluorinated d -swainsonine derivatives and their enantiomers have been designed based on initial docking calculations, and synthesized from enantiomeric ribose-derived aldehydes, respectively. Glycosidase inhibition assay of these derivatives with d -swainsonine (1 ) and l -swainsonine (ent -1d -swainsonine derivatives with C-8 configurations as R (α) showed specific and potent inhibitions of jack bean α-mannosidase (model enzyme of Golgi α-mannosidase II); whereas their enantiomers with C-8 configurations as S (β) were powerful and selective α-l -rhamnosidase inhibitors. Molecular docking calculations found the C-6 fluorinatedd -swainsonine derivatives 21 , 24 and 25 with highly coincident binding conformations with d -swainsonine (1 ) in their interactions with the active site of α-mannosidase (PDB ID: 1HWW ). Reliability of the docking results were confirmed by Molecular Dynamics (MD) simulation. Additionally, solid interactions with residues Gln-392 and Tyr-393 in the active site of α-l -rhamnosidase (PDB ID: 3W5N ) were proved to be vital for potent α-l -rhamnosidase inhibitions of the l -swainsonine derivatives. The role of C-6 fluorines in swainsonine derivatives well demonstrated the “mimic effect” of fluorine to hydrogen by minimal influence on the binding conformations and effective compensation for any possible lost interactions. This work contributes to a comprehensive understanding of the structure-activity relationship (SAR) of the fluorinated swainsonines and ever reported branched swainsonines, and has laid good foundation for development of more potent α-mannosidase and α-l -rhamnosidase inhibitors.
中文翻译:

对映体 C-6 氟化苦马豆素衍生物作为 α-甘露糖苷酶和 α-l-鼠李糖苷酶的高选择性和有效抑制剂:设计、合成和构效关系研究
基于初始对接计算设计了六种 C-6 氟化 d-苦马红素衍生物及其对映异构体,并分别由对映体核糖衍生的醛合成。以 d-苦马豆素 (1) 和 l-苦马豆素 (ent-1) 作为对比的这些衍生物的糖苷酶抑制试验发现,以 C-8 构型为 R (α) 的 C-6 氟化 d-苦马豆素衍生物显示出对菠萝豆 α-甘露糖苷酶 (高尔基体 α-甘露糖苷酶 II 的模型酶)的特异性和有效抑制作用;而它们的对映异构体以 C-8 构型为 S (β) 是强大的选择性 α-l-鼠李糖苷酶抑制剂。分子对接计算发现,C-6 氟化dd-苦马豆素衍生物 21、24 和 25 在与 α-甘露糖苷酶活性位点 (PDB ID: 1HWW) 的相互作用中与 d-苦马豆素 (1) 具有高度重合的结合构象。分子动力学 (MD) 模拟证实了对接结果的可靠性。此外,与 α-l-鼠李糖苷酶 (PDB ID: 3W5N) 活性位点的残基 Gln-392 和 Tyr-393 的固体相互作用被证明对 l-苦马豆素衍生物的有效 α-l-鼠李糖苷酶抑制至关重要。C-6 氟在苦马豆素衍生物中的作用很好地证明了氟对氢的“模拟效应”,对结合构象的影响最小,并有效补偿任何可能的相互作用损失。这项工作有助于全面了解氟化苦马豆素和曾经报道的支链苦马豆素的构效关系 (SAR),并为开发更有效的 α-甘露糖苷酶和 α-l-鼠李糖苷酶抑制剂奠定了良好的基础。
更新日期:2024-11-12
中文翻译:

对映体 C-6 氟化苦马豆素衍生物作为 α-甘露糖苷酶和 α-l-鼠李糖苷酶的高选择性和有效抑制剂:设计、合成和构效关系研究
基于初始对接计算设计了六种 C-6 氟化 d-苦马红素衍生物及其对映异构体,并分别由对映体核糖衍生的醛合成。以 d-苦马豆素 (1) 和 l-苦马豆素 (ent-1) 作为对比的这些衍生物的糖苷酶抑制试验发现,以 C-8 构型为 R (α) 的 C-6 氟化 d-苦马豆素衍生物显示出对菠萝豆 α-甘露糖苷酶 (高尔基体 α-甘露糖苷酶 II 的模型酶)的特异性和有效抑制作用;而它们的对映异构体以 C-8 构型为 S (β) 是强大的选择性 α-l-鼠李糖苷酶抑制剂。分子对接计算发现,C-6 氟化dd-苦马豆素衍生物 21、24 和 25 在与 α-甘露糖苷酶活性位点 (PDB ID: 1HWW) 的相互作用中与 d-苦马豆素 (1) 具有高度重合的结合构象。分子动力学 (MD) 模拟证实了对接结果的可靠性。此外,与 α-l-鼠李糖苷酶 (PDB ID: 3W5N) 活性位点的残基 Gln-392 和 Tyr-393 的固体相互作用被证明对 l-苦马豆素衍生物的有效 α-l-鼠李糖苷酶抑制至关重要。C-6 氟在苦马豆素衍生物中的作用很好地证明了氟对氢的“模拟效应”,对结合构象的影响最小,并有效补偿任何可能的相互作用损失。这项工作有助于全面了解氟化苦马豆素和曾经报道的支链苦马豆素的构效关系 (SAR),并为开发更有效的 α-甘露糖苷酶和 α-l-鼠李糖苷酶抑制剂奠定了良好的基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号