当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A catalytic atroposelective Friedel–Crafts alkylation to access axially chiral C2-arylindoles via dynamic kinetic resolutions
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-13 , DOI: 10.1039/d4qo01611d Jiang Deng, Wei Li, Chenhao Zhou, Zhiming Li, Haibo Zhou, Junyuan Yan, Zhouyu Wang, Shan Qian, Xiao-Long He
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-13 , DOI: 10.1039/d4qo01611d Jiang Deng, Wei Li, Chenhao Zhou, Zhiming Li, Haibo Zhou, Junyuan Yan, Zhouyu Wang, Shan Qian, Xiao-Long He
|
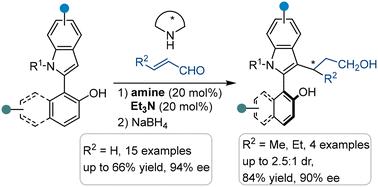
Indole-based atropisomers have emerged as an important backbone in drug discovery and novel catalyst/ligand design. However, the development of asymmetric synthesis of axially chiral 2-arylindoles is sluggish due to their very low rotation barrier. Herein, we developed an amine-catalyzed atroposelective F–C alkylation of rotation-free 2-arylindoles with α,β-unsaturated aldehydes via the DKR process. Various axially chiral 2-arylindoles were obtained with good results. Besides, a promising chiral phosphine was derived from the obtained atropisomers.
中文翻译:

一种催化性萎缩选择性 Friedel-Crafts 烷基化反应,通过动态动力学分辨率获得轴向手性 C2-芳吲哚
基于吲哚的促异构体已成为药物发现和新型催化剂/配体设计的重要支柱。然而,由于轴向手性 2-芳吲哚的旋转势垒非常低,因此轴向手性 2-芳吲哚的不对称合成的发展缓慢。在此,我们通过 DKR 工艺开发了一种胺催化的无旋转 2-芳吲哚与 α,β-不饱和醛的无旋选择性 F-C 烷基化。获得了各种轴向手性 2-芳吲哚,结果良好。此外,从获得的促性异构体衍生出一种有前途的手性膦。
更新日期:2024-11-13
中文翻译:

一种催化性萎缩选择性 Friedel-Crafts 烷基化反应,通过动态动力学分辨率获得轴向手性 C2-芳吲哚
基于吲哚的促异构体已成为药物发现和新型催化剂/配体设计的重要支柱。然而,由于轴向手性 2-芳吲哚的旋转势垒非常低,因此轴向手性 2-芳吲哚的不对称合成的发展缓慢。在此,我们通过 DKR 工艺开发了一种胺催化的无旋转 2-芳吲哚与 α,β-不饱和醛的无旋选择性 F-C 烷基化。获得了各种轴向手性 2-芳吲哚,结果良好。此外,从获得的促性异构体衍生出一种有前途的手性膦。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号