当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intermolecular defluorinative 1,2-diamination of fluoroalkenyl iodides with sulfonamides: synthesis of acyclic and cyclic fluorinated 1,2-enediamines
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-13 , DOI: 10.1039/d4qo01913j Xue-Qiang Chu, Yu-Lan Chen, Chi Zhang, Xue-Ying Huang, Yu Ding, Danhua Ge, Zhi-Liang Shen
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-13 , DOI: 10.1039/d4qo01913j Xue-Qiang Chu, Yu-Lan Chen, Chi Zhang, Xue-Ying Huang, Yu Ding, Danhua Ge, Zhi-Liang Shen
|
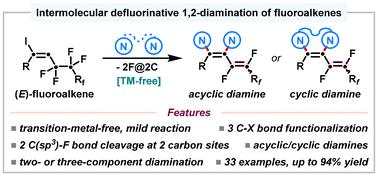
Alkene diamination represents one of the most straightforward strategies for 1,2-diamine derivatives synthesis. However, transition-metal-free 1,2-diamination of fluoroalkenes has been less studied. Here we report an intermolecular defluorinative vicinal diamination protocol employing easily prepared fluoroalkenyl iodides and sulfonamides for the efficient synthesis of a series of fluoroalkenyl-containing acyclic and cyclic 1,2-enediamines. The multiple halogen atoms (one iodine atom and two fluorine atoms) present on functionalized fluoroalkenes function as detachable “activators”, facilitating C–N bond formation under conditions that are devoid of transition metals, electricity, and photoirradiation. This method overcomes the need for additional oxidants required by traditional methods and exhibits good functional group tolerance and excellent scalability. The synthetic versatility is also demonstrated in the late-stage modification of biologically active molecules.
中文翻译:

氟烯基碘化物与磺胺类药物的分子间脱氟 1,2-二胺化:无环和环状氟化 1,2-烯二胺的合成
烯烃二胺化是 1,2-二胺衍生物合成的最直接策略之一。然而,氟烯烃的无过渡金属 1,2-二胺化反应研究较少。在这里,我们报告了一种分子间脱氟邻二胺化方案,该方案采用易于制备的氟烯基碘化物和磺胺类药物来高效合成一系列含氟烯基的无环和环状 1,2-烯二胺。功能化氟烯烃上的多个卤素原子(一个碘原子和两个氟原子)起着可拆卸“活化剂”的作用,在没有过渡金属、电和光照射的条件下促进 C-N 键的形成。该方法克服了传统方法所需的额外氧化剂需求,并表现出良好的官能团耐受性和出色的可扩展性。合成的多功能性也体现在生物活性分子的后期修饰中。
更新日期:2024-11-15
中文翻译:

氟烯基碘化物与磺胺类药物的分子间脱氟 1,2-二胺化:无环和环状氟化 1,2-烯二胺的合成
烯烃二胺化是 1,2-二胺衍生物合成的最直接策略之一。然而,氟烯烃的无过渡金属 1,2-二胺化反应研究较少。在这里,我们报告了一种分子间脱氟邻二胺化方案,该方案采用易于制备的氟烯基碘化物和磺胺类药物来高效合成一系列含氟烯基的无环和环状 1,2-烯二胺。功能化氟烯烃上的多个卤素原子(一个碘原子和两个氟原子)起着可拆卸“活化剂”的作用,在没有过渡金属、电和光照射的条件下促进 C-N 键的形成。该方法克服了传统方法所需的额外氧化剂需求,并表现出良好的官能团耐受性和出色的可扩展性。合成的多功能性也体现在生物活性分子的后期修饰中。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号