当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Free-Standing Boron-Doped Diamond Grid Electrode for Fundamental Spectroelectrochemistry
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.analchem.4c00906 Hannah K. Patenaude, Nastasija Damjanovic, Jason Rakos, Dustyn C. Weber, Aaron I. Jacobs, Samuel A. Bryan, Amanda M. Lines, William R. Heineman, Shirmir D. Branch, Cory A. Rusinek
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.analchem.4c00906 Hannah K. Patenaude, Nastasija Damjanovic, Jason Rakos, Dustyn C. Weber, Aaron I. Jacobs, Samuel A. Bryan, Amanda M. Lines, William R. Heineman, Shirmir D. Branch, Cory A. Rusinek
|
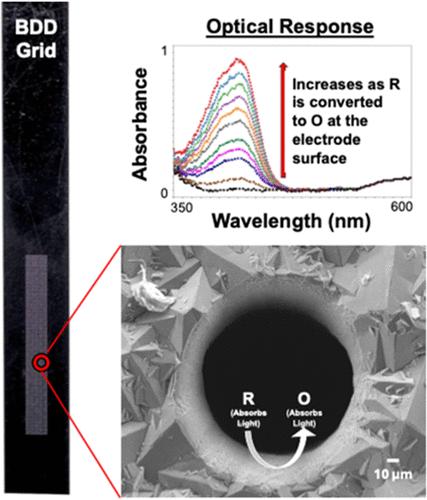
Spectroelectrochemistry (SEC) is a powerful technique that enables a variety of redox properties to be studied, including formal potential (Eo), thermodynamic values (ΔG, ΔH, ΔS), diffusion coefficient (D), electron transfer stoichiometry (n), and others. SEC requires an electrode which light can pass through while maintaining sufficient electrical conductivity. This has been traditionally composed of metal or metal oxide films atop transparent substrates like glass, quartz, or metallic mesh. Robust electrode materials like boron-doped diamond (BDD) could help expand the environments in which SEC can be performed, but most designs are limited to thin films (∼100–200 nm) on transparent substrates less resilient than free-standing BDD. This work presents a free-standing BDD grid electrode (G-BDD) for fundamental SEC measurements, using the well-characterized Fe(CN)63–/4– redox couple as proof-of-concept. With a combination of cyclic voltammetry (CV), thin-layer SEC, and chronoabsorptometry, several of the redox properties mentioned above were calculated and compared. For Eo′, n, and D, similar results were obtained when comparing the CV [Eo′ = +0.279 (±0.002) V vs Ag/AgCl; n = 0.97; D = 4.1 × 10–6 cm2·s–1] and SEC [Eo′ = +0.278 (±0.001) V vs Ag/AgCl; n = 0.91; D = 5.2 × 10–6 cm2·s–1] techniques. Both values align with what has been previously reported. To calculate D from the SEC data, modification of the classical equation used in chronoabsorptometry was required to accommodate the G-BDD electrode geometry. Overall, this work expands on the applicability of SEC techniques and BDD as a versatile electrode material.
中文翻译:

用于基础光谱电化学的独立式掺硼金刚石栅电极
光谱电化学 (SEC) 是一种强大的技术,可以研究各种氧化还原特性,包括形式电位 (EO)、热力学值(ΔG、ΔH、ΔS)、扩散系数 (D)、电子转移化学计量 (n) 等。SEC 需要一个光可以穿过的电极,同时保持足够的导电性。传统上,它由玻璃、石英或金属网等透明基材上的金属或金属氧化膜组成。像掺硼金刚石 (BDD) 这样的坚固电极材料可以帮助扩展可以执行 SEC 的环境,但大多数设计仅限于透明衬底上的薄膜 (∼100–200 nm),其弹性不如独立式 BDD。这项工作提出了一种用于基本 SEC 测量的独立式 BDD 网格电极 (G-BDD),使用表征良好的 Fe(CN)63–/4– 氧化还原对作为概念验证。结合循环伏安法 (CV)、薄层 SEC 和计时吸收法,计算并比较了上述几种氧化还原特性。对于 Eo′、n 和 D,在比较 CV [Eo′ = +0.279 (±0.002) V 与 Ag/AgCl 时,获得了相似的结果;n = 0.97;D = 4.1 × 10–6 cm2·s–1] 和 SEC [Eo′ = +0.278 (±0.001) V vs Ag/AgCl;n = 0.91;D = 5.2 × 10–6 cm2·s–1] 技术。这两个值都与之前报告的值一致。 为了从 SEC 数据计算 D,需要修改计时吸收测定中使用的经典方程,以适应 G-BDD 电极几何形状。总体而言,这项工作扩展了 SEC 技术和 BDD 作为多功能电极材料的适用性。
更新日期:2024-11-13
中文翻译:

用于基础光谱电化学的独立式掺硼金刚石栅电极
光谱电化学 (SEC) 是一种强大的技术,可以研究各种氧化还原特性,包括形式电位 (EO)、热力学值(ΔG、ΔH、ΔS)、扩散系数 (D)、电子转移化学计量 (n) 等。SEC 需要一个光可以穿过的电极,同时保持足够的导电性。传统上,它由玻璃、石英或金属网等透明基材上的金属或金属氧化膜组成。像掺硼金刚石 (BDD) 这样的坚固电极材料可以帮助扩展可以执行 SEC 的环境,但大多数设计仅限于透明衬底上的薄膜 (∼100–200 nm),其弹性不如独立式 BDD。这项工作提出了一种用于基本 SEC 测量的独立式 BDD 网格电极 (G-BDD),使用表征良好的 Fe(CN)63–/4– 氧化还原对作为概念验证。结合循环伏安法 (CV)、薄层 SEC 和计时吸收法,计算并比较了上述几种氧化还原特性。对于 Eo′、n 和 D,在比较 CV [Eo′ = +0.279 (±0.002) V 与 Ag/AgCl 时,获得了相似的结果;n = 0.97;D = 4.1 × 10–6 cm2·s–1] 和 SEC [Eo′ = +0.278 (±0.001) V vs Ag/AgCl;n = 0.91;D = 5.2 × 10–6 cm2·s–1] 技术。这两个值都与之前报告的值一致。 为了从 SEC 数据计算 D,需要修改计时吸收测定中使用的经典方程,以适应 G-BDD 电极几何形状。总体而言,这项工作扩展了 SEC 技术和 BDD 作为多功能电极材料的适用性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号