Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into Target Gas–Oxygen Interactions in Highly Sensitive Gas Sensors Using Data-Driven Methods
ACS Sensors ( IF 8.2 ) Pub Date : 2024-11-12 , DOI: 10.1021/acssensors.4c01284 Kyusung Kim, Phuwadej Pornaroontham, Hojung Yun, Sungmin Kim, Pilgyu Choi, Yoshitake Masuda
ACS Sensors ( IF 8.2 ) Pub Date : 2024-11-12 , DOI: 10.1021/acssensors.4c01284 Kyusung Kim, Phuwadej Pornaroontham, Hojung Yun, Sungmin Kim, Pilgyu Choi, Yoshitake Masuda
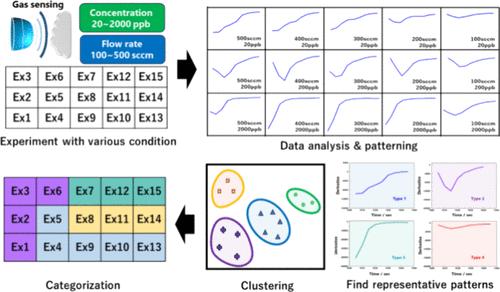
|
In the gas-sensing mechanism of a metal-oxide-semiconductor (n-type) gas sensor, oxygen adsorption or desorption on the oxide surface leads to an increase or decrease in the resistance of the gas sensor system. Additionally, oxygen can be adsorbed again at the location where initially adsorbed oxygen reacted with the target gas. Thus, the adsorption–desorption equilibrium of the reducing gas on the oxide surface is a significant factor in determining the sensitivity and reaction rate. In particular, for ultralow-concentration gas measurements, the relative concentration of oxygen was very high. To design an ultrasensitive gas sensor, not only the reaction of the target gas but also the competing reaction between the target gas and oxygen must be considered. Although qualitative investigations of these complex relationships have been performed according to the gas concentration and flow rate, reliable quantitative results are limited. In this study, a quantitative approach was used to understand the correlation between oxygen and a target gas by applying data analysis methods. We investigated the behavior of oxygen and the target molecules depending on the gas concentration and flow rate using the parts per billion level of the acetone gas sensor. Initial response data according to various detection conditions were processed using principal component analysis and K-means clustering; as a result, four types of reaction behaviors were inferred for 15 types of reaction conditions. Furthermore, the response time, depending on the detection conditions, can be distinguished using the suggested categorization. Our investigation suggests a possibility beyond simple optimization through the data analysis of the gas-sensing results.
中文翻译:

使用数据驱动方法深入了解高灵敏度气体传感器中的目标气体-氧气相互作用
在金属氧化物半导体(n 型)气体传感器的气体传感机构中,氧在氧化物表面的吸附或解吸会导致气体传感器系统的电阻增加或减少。此外,氧气可以在最初吸附的氧气与目标气体反应的位置再次吸附。因此,还原气体在氧化物表面的吸附-解吸平衡是决定灵敏度和反应速率的重要因素。特别是,对于超低浓度气体测量,氧气的相对浓度非常高。要设计超灵敏气体传感器,不仅要考虑目标气体的反应,还必须考虑目标气体和氧气之间的竞争反应。尽管已经根据气体浓度和流速对这些复杂关系进行了定性研究,但可靠的定量结果有限。在这项研究中,通过应用数据分析方法,使用定量方法来了解氧气和目标气体之间的相关性。我们使用丙酮气体传感器的十亿分之一水平研究了氧气和目标分子的行为,具体取决于气体浓度和流速。使用主成分分析和 K-means 聚类处理根据各种检测条件的初始响应数据;结果,针对 15 种反应条件推断出 4 种类型的反应行为。此外,根据检测条件,可以使用建议的分类来区分响应时间。我们的调查表明,通过对气体传感结果的数据分析,除了简单的优化之外,还有一种可能性。
更新日期:2024-11-13
中文翻译:

使用数据驱动方法深入了解高灵敏度气体传感器中的目标气体-氧气相互作用
在金属氧化物半导体(n 型)气体传感器的气体传感机构中,氧在氧化物表面的吸附或解吸会导致气体传感器系统的电阻增加或减少。此外,氧气可以在最初吸附的氧气与目标气体反应的位置再次吸附。因此,还原气体在氧化物表面的吸附-解吸平衡是决定灵敏度和反应速率的重要因素。特别是,对于超低浓度气体测量,氧气的相对浓度非常高。要设计超灵敏气体传感器,不仅要考虑目标气体的反应,还必须考虑目标气体和氧气之间的竞争反应。尽管已经根据气体浓度和流速对这些复杂关系进行了定性研究,但可靠的定量结果有限。在这项研究中,通过应用数据分析方法,使用定量方法来了解氧气和目标气体之间的相关性。我们使用丙酮气体传感器的十亿分之一水平研究了氧气和目标分子的行为,具体取决于气体浓度和流速。使用主成分分析和 K-means 聚类处理根据各种检测条件的初始响应数据;结果,针对 15 种反应条件推断出 4 种类型的反应行为。此外,根据检测条件,可以使用建议的分类来区分响应时间。我们的调查表明,通过对气体传感结果的数据分析,除了简单的优化之外,还有一种可能性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号