当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed (Asymmetric) Annulation of Silacyclobutanes with Bicyclic Olefins via C–Si Bond Activation
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acscatal.4c05675 Shengbo Xu, Fen Wang, Xingwei Li
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acscatal.4c05675 Shengbo Xu, Fen Wang, Xingwei Li
|
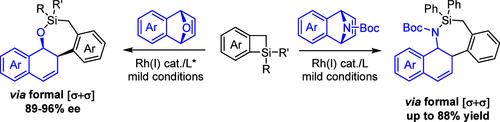
The carbon-to-silicon switch gives rise to silacycles that offer eminent biological and photophysical properties. Access to chiral silacycles, especially midsized ones, via intermolecular coupling remains a considerable challenge due to limited synthetic methods. Herein, rhodium(I)-catalyzed annulations between benzosilacyclobutenes (SCBs) and bicyclic olefins are presented. A series of stable seven-membered chiral silacycles have been accessed in high enantioselectivity via the enantioselective [4 + 3] annulation between SCBs and 7-oxabenzonorbornadienes via a formal [2σ + 2σ] C–C and O–Si coupling. The mechanism of the enantioselective [4 + 3] annulation between SCBs and 7-oxabenzonorbornadienes has been investigated, where C–Si oxidative addition of the SCB has been established as the turnover-limiting step.
中文翻译:

铑催化(不对称)硅环丁烷与双环烯烃的环化,通过 C-Si 键活化
碳硅转换产生了具有突出生物和光物理特性的硅环。由于合成方法有限,通过分子间偶联获得手性硅烷环,尤其是中型硅烷环仍然是一个相当大的挑战。在此,提出了苯并硅环丁烯 (SCB) 和双环烯烃之间的铑 (I) 催化环化。通过 SCB 和 7-恶杂苯并硼二烯之间的对映选择性 [4 + 3] 环化,通过正式的 [2σ + 2σ] C-C 和 O-Si 偶联,以高对映选择性获得一系列稳定的七元手性硅烷环。已经研究了 SCB 和 7-恶杂苯并硼二烯之间的对映选择性 [4 + 3] 环化机制,其中 SCB 的 C-Si 氧化加成已被确定为周转限制步骤。
更新日期:2024-11-13
中文翻译:

铑催化(不对称)硅环丁烷与双环烯烃的环化,通过 C-Si 键活化
碳硅转换产生了具有突出生物和光物理特性的硅环。由于合成方法有限,通过分子间偶联获得手性硅烷环,尤其是中型硅烷环仍然是一个相当大的挑战。在此,提出了苯并硅环丁烯 (SCB) 和双环烯烃之间的铑 (I) 催化环化。通过 SCB 和 7-恶杂苯并硼二烯之间的对映选择性 [4 + 3] 环化,通过正式的 [2σ + 2σ] C-C 和 O-Si 偶联,以高对映选择性获得一系列稳定的七元手性硅烷环。已经研究了 SCB 和 7-恶杂苯并硼二烯之间的对映选择性 [4 + 3] 环化机制,其中 SCB 的 C-Si 氧化加成已被确定为周转限制步骤。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号