当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extraction mechanism of phenolic compounds by a choline chloride/glycerol solvent: DFT and molecular dynamics studies
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4cp03453h Lan Yi, Jinwen Wang, Jixing Liu, Hao Luo, Xiaoqin Wu, Wen-Ying Li
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4cp03453h Lan Yi, Jinwen Wang, Jixing Liu, Hao Luo, Xiaoqin Wu, Wen-Ying Li
|
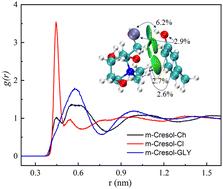
The mechanism of a solvent consisting of choline chloride and glycerol (ChCl/GLY) for extracting phenolic compounds from coal tar was theoretically studied using density functional theory calculations and molecular dynamics simulations. The thermodynamic properties, interaction essence, and molecular dynamics properties of the extraction system were investigated, as well as the influence of ChCl/GLY on the vibration spectra of phenolic compounds. The results show that the solvation free energy of phenolic compounds in ChCl/GLY is more negative than that in coal tar, leading to the spontaneous transfer of phenolic compounds from coal tar to ChCl/GLY. The electrostatic and dispersion interactions between phenolic compounds and ChCl/GLY have similar significance in the extraction process, with interaction energies ranging from −46 to −53 kJ mol−1. The mixing of phenolic compounds with ChCl/GLY has minimal impact on their internal molecular structure, however, it does reduce the diffusion coefficients of each component in ChCl/GLY and shortens the lifetime of hydrogen bonds in both phenolic compounds and ChCl/GLY. The first shell of each phenolic compound is surrounded by 1.15 chloride ions. Following dissolving in ChCl/GLY, the stretching vibration peaks of phenolic compounds, namely the –OH and C–H/–CH3 regions, undergo a shift. The results enhance comprehension of the extraction process of phenolic compounds by DES.
中文翻译:

氯化胆碱/甘油溶剂提取酚类化合物的机理:DFT 和分子动力学研究
采用密度泛函理论计算和分子动力学模拟,从理论上研究了由氯化胆碱和甘油组成的溶剂 (ChCl/GLY) 从煤焦油中提取酚类化合物的机理。研究了提取体系的热力学性质、相互作用本质和分子动力学性质,以及 ChCl/GLY 对酚类化合物振动光谱的影响。结果表明,酚类化合物在 ChCl/GLY 中的溶剂化自由能比煤焦油中的溶剂化自由能更负,导致酚类化合物从煤焦油自发转移到 ChCl/GLY。酚类化合物和 ChCl/GLY 之间的静电和分散相互作用在提取过程中具有相似的意义,相互作用能范围为 -46 至 -53 kJ mol-1。酚类化合物与 ChCl/GLY 的混合对其内部分子结构的影响很小,但它确实降低了 ChCl/GLY 中各组分的扩散系数,并缩短了酚类化合物和 ChCl/GLY 中氢键的寿命。每种酚类化合物的第一层被 1.15 个氯离子包围。溶解在 ChCl/GLY 中后,酚类化合物的拉伸振动峰,即 –OH 和 C–H/–CH 3 区域发生偏移。结果增强了对 DES 提取酚类化合物过程的理解。
更新日期:2024-11-12
中文翻译:

氯化胆碱/甘油溶剂提取酚类化合物的机理:DFT 和分子动力学研究
采用密度泛函理论计算和分子动力学模拟,从理论上研究了由氯化胆碱和甘油组成的溶剂 (ChCl/GLY) 从煤焦油中提取酚类化合物的机理。研究了提取体系的热力学性质、相互作用本质和分子动力学性质,以及 ChCl/GLY 对酚类化合物振动光谱的影响。结果表明,酚类化合物在 ChCl/GLY 中的溶剂化自由能比煤焦油中的溶剂化自由能更负,导致酚类化合物从煤焦油自发转移到 ChCl/GLY。酚类化合物和 ChCl/GLY 之间的静电和分散相互作用在提取过程中具有相似的意义,相互作用能范围为 -46 至 -53 kJ mol-1。酚类化合物与 ChCl/GLY 的混合对其内部分子结构的影响很小,但它确实降低了 ChCl/GLY 中各组分的扩散系数,并缩短了酚类化合物和 ChCl/GLY 中氢键的寿命。每种酚类化合物的第一层被 1.15 个氯离子包围。溶解在 ChCl/GLY 中后,酚类化合物的拉伸振动峰,即 –OH 和 C–H/–CH 3 区域发生偏移。结果增强了对 DES 提取酚类化合物过程的理解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号