当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hemilabile P,N-ligand-assisted gold catalyzed Hiyama allylation to access allylarenes and 1,4-diene derivatives
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4qo01879f Yuanhao He, Wanping Ma, Yu Zhong, Yanfei Hu, Mingou Li, Fen Zhao, Zhonghua Xia, Baomin Fan
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4qo01879f Yuanhao He, Wanping Ma, Yu Zhong, Yanfei Hu, Mingou Li, Fen Zhao, Zhonghua Xia, Baomin Fan
|
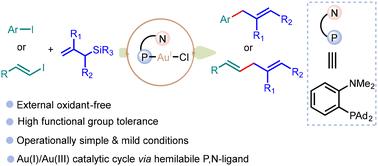
Oxidant-free gold-catalyzed reactions are emerging as a novel synthetic tool for organic transformations. Herein, we report a hemilabile P,N-ligand-assisted gold-catalyzed Hiyama cross coupling reaction of aryl and alkenyl iodides with allylsilanes. The reaction proceeds smoothly without an external oxidant under mild conditions, providing access to a series of allylarenes and 1,4-diene derivatives in good to excellent yields. This transformation features a broad substrate scope, good functional group tolerance, and compatibility with heteroaromatic substrates. The (P,N) ligand MeDalphos-facilitated gold-catalyzed process presents a new approach for the synthesis of allylarenes and 1,4-diene derivatives. Moreover, mechanistic investigations including NMR and mass spectrometric studies strongly support the proposed mechanism of the reaction.
中文翻译:

Hemilabile P,N-配体辅助金催化 Hiyama 烯丙基化以获得烯丙烯和 1,4-二烯衍生物
无氧化剂金催化反应正在成为一种新型的有机转化合成工具。在此,我们报道了芳基和烯基碘化物与烯基硅烷的半 P,N-配体辅助金催化的 Hiyama 交叉偶联反应。在温和的条件下,在没有外部氧化剂的情况下,反应顺利进行,以良好到极好的收率获得一系列烯丙烯和 1,4-二烯衍生物。这种转化具有广泛的底物范围、良好的官能团耐受性以及与杂芳烃底物的相容性。(P,N) 配体 MeDalphos 促进的金催化工艺为烯丙烯和 1,4-二烯衍生物的合成提供了一种新方法。此外,包括 NMR 和质谱研究在内的机理研究强烈支持所提出的反应机理。
更新日期:2024-11-12
中文翻译:

Hemilabile P,N-配体辅助金催化 Hiyama 烯丙基化以获得烯丙烯和 1,4-二烯衍生物
无氧化剂金催化反应正在成为一种新型的有机转化合成工具。在此,我们报道了芳基和烯基碘化物与烯基硅烷的半 P,N-配体辅助金催化的 Hiyama 交叉偶联反应。在温和的条件下,在没有外部氧化剂的情况下,反应顺利进行,以良好到极好的收率获得一系列烯丙烯和 1,4-二烯衍生物。这种转化具有广泛的底物范围、良好的官能团耐受性以及与杂芳烃底物的相容性。(P,N) 配体 MeDalphos 促进的金催化工艺为烯丙烯和 1,4-二烯衍生物的合成提供了一种新方法。此外,包括 NMR 和质谱研究在内的机理研究强烈支持所提出的反应机理。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号