当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modular dehydrogenative cross-nucleophile coupling for direct construction of tetrasubstituted carbons
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4qo01641f Peng Wang, Qiang Wang, Meng Wang, Liang Wang, Lubin Xu, Xiong-Li Liu, Fangzhi Hu, Shuai-Shuai Li
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4qo01641f Peng Wang, Qiang Wang, Meng Wang, Liang Wang, Lubin Xu, Xiong-Li Liu, Fangzhi Hu, Shuai-Shuai Li
|
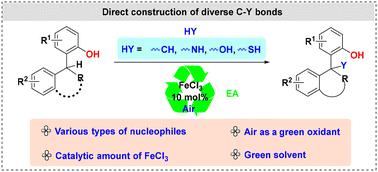
The development of a unified platform for the cross-nucleophile coupling of tertiary C(sp3)–H bonds with diverse nucleophiles, ideally using readily available catalysts and inexpensive and green oxidants, remains a compelling challenge. We now asserted the FeCl3-catalyzed cross-dehydrogenative coupling reaction between ortho-hydroxyl benzylic tertiary C(sp3)–H bonds and diverse nucleophiles under an air atmosphere for the rapid construction of various types of C–Y (Y = C, N, O, S) bonds. This reaction enables the modular dehydrogenative cross-nucleophile coupling for the direct construction of tetrasubstituted carbons featuring broad substrate tolerance and environmental friendliness. In addition, the highly functionalized benzofuroindoline core could also be facilely synthesized by the direct transformation from the in situ reduction/cyclization or nucleophilic addition/cyclization of 3,3-disubstituted lactams.
中文翻译:

用于直接构建四取代碳的模块化脱氢交叉亲核试剂偶联
开发一个统一的平台,用于叔级 C(sp3)-H 键与多种亲核试剂的交叉亲核试剂偶联,理想情况下使用现成的催化剂和廉价的绿色氧化剂,仍然是一个引人注目的挑战。我们现在断言,在空气气氛下,邻羟基苄基叔 C(sp3)-H 键和多种亲核试剂之间的 FeCl3 催化的交叉脱氢偶联反应,用于快速构建各种类型的 C-Y (Y = C, N, O, S) 键。该反应实现了模块化脱氢交叉亲核试剂偶联,用于直接构建具有广泛底物耐受性和环境友好性的四取代碳。此外,高度功能化的苯并呋吲哚啉核心也可以通过 3,3-二取代内酰胺的原位还原/环化或亲核加成/环化直接转化而轻松合成。
更新日期:2024-11-12
中文翻译:

用于直接构建四取代碳的模块化脱氢交叉亲核试剂偶联
开发一个统一的平台,用于叔级 C(sp3)-H 键与多种亲核试剂的交叉亲核试剂偶联,理想情况下使用现成的催化剂和廉价的绿色氧化剂,仍然是一个引人注目的挑战。我们现在断言,在空气气氛下,邻羟基苄基叔 C(sp3)-H 键和多种亲核试剂之间的 FeCl3 催化的交叉脱氢偶联反应,用于快速构建各种类型的 C-Y (Y = C, N, O, S) 键。该反应实现了模块化脱氢交叉亲核试剂偶联,用于直接构建具有广泛底物耐受性和环境友好性的四取代碳。此外,高度功能化的苯并呋吲哚啉核心也可以通过 3,3-二取代内酰胺的原位还原/环化或亲核加成/环化直接转化而轻松合成。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号