当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PIFA-mediated cyclization of methyl(2-(1-phenylvinyl)phenyl)sulfane for the concise, flexible, and scalable de novo synthesis of C3-arylated benzo[b]thiophenes
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4qo01589d Xinya Liu, Olivier Provot, Christine Tran, Jean François Soulé, Abdallah Hamze
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4qo01589d Xinya Liu, Olivier Provot, Christine Tran, Jean François Soulé, Abdallah Hamze
|
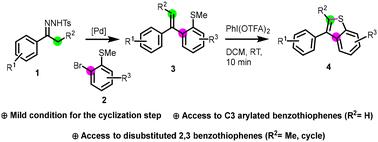
The well-decorated arylated benzo[b]thiophene scaffold is a pivotal structural motif with diverse applications in medicinal chemistry. This paper outlines a de novo synthesis of C3-arylated benzo[b]thiophenes, showcasing its flexibility and efficiency. The methodology involves Pd-catalyzed coupling of N-tosylhydrazones with (2-bromophenyl)(methyl)sulfane derivatives, followed by cyclization under mild conditions using [bis-(trifluoroacetoxy)iodo]benzene (PIFA) to yield the desired C3-arylated benzo[b]thiophenes. Substrate scope investigations and a gram-scale reaction underscore this protocol's synthetic potential and scalability. The developed approach offers a concise and versatile strategy for accessing this important class of compounds, providing a valuable tool for medicinal chemistry and pharmaceutical research.
中文翻译:

PIFA 介导的甲基(2-(1-苯基)苯基)硫烷环化,用于 C3-芳基苯并[b]噻吩的简洁、灵活且可扩展的从头合成
装饰精美的芳基苯并[b]噻吩支架是一个关键的结构基序,在药物化学中有多种应用。本文概述了 C3-芳基苯并[b]噻吩的从头合成,展示了其灵活性和效率。该方法包括 N-甲苯腙与(2-溴苯基)(甲基)硫烷衍生物的 Pd 催化偶联,然后在温和条件下使用 [双-(三氟乙酰氧基)碘]苯 (PIFA) 进行环化,以产生所需的 C3-芳基苯并[b]噻吩。底物范围研究和克级反应强调了该方案的合成潜力和可扩展性。开发的方法为获取这类重要的化合物提供了一种简洁而通用的策略,为药物化学和药物研究提供了有价值的工具。
更新日期:2024-11-12
中文翻译:

PIFA 介导的甲基(2-(1-苯基)苯基)硫烷环化,用于 C3-芳基苯并[b]噻吩的简洁、灵活且可扩展的从头合成
装饰精美的芳基苯并[b]噻吩支架是一个关键的结构基序,在药物化学中有多种应用。本文概述了 C3-芳基苯并[b]噻吩的从头合成,展示了其灵活性和效率。该方法包括 N-甲苯腙与(2-溴苯基)(甲基)硫烷衍生物的 Pd 催化偶联,然后在温和条件下使用 [双-(三氟乙酰氧基)碘]苯 (PIFA) 进行环化,以产生所需的 C3-芳基苯并[b]噻吩。底物范围研究和克级反应强调了该方案的合成潜力和可扩展性。开发的方法为获取这类重要的化合物提供了一种简洁而通用的策略,为药物化学和药物研究提供了有价值的工具。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号