当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh-Catalysed hydroacyloxylation of cyclopropenes: regio- and diastereoselective synthesis of acyloxycyclopropanes
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4qo01810a Ángel Manu Martínez, Gema Domínguez, Inés Alonso, Marta Palaín, Javier Pérez-Castells
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4qo01810a Ángel Manu Martínez, Gema Domínguez, Inés Alonso, Marta Palaín, Javier Pérez-Castells
|
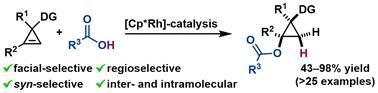
Acyloxycyclopropanes are essential building blocks in biological and medicinal chemistry. However, their accessibility is severely limited by the need to pre-synthesise highly reactive cyclopropane derivatives. Herein, we report an efficient stereoselective synthesis of polysubstituted acyloxycyclopropanes via Cp*Rh(III)-catalysed hydroacyloxylation of cyclopropenes. This catalytic system allows the regio-, facial-, and syn-selective addition of various inexpensive and commercially available carboxylic acids to the double bond of cyclopropene. This protocol is carried out under very mild conditions and shows a wide group tolerance, providing undescribed acyloxycyclopropanes with very good yields and exclusive diastereoselectivity. Furthermore, the reaction can be carried out intramolecularly towards interesting cyclopropyl spiro-lactones. Isolation of key organometallic complexes, NMR monitoring studies, HMRS experiments and DFT calculations have revealed significant mechanistic insights.
中文翻译:

环丙烯的 Rh 催化氢酰氧基化反应:酰氧基环丙烷的区域和非对映选择性合成
酰氧基环丙烷是生物和药物化学中必不可少的组成部分。然而,由于需要预合成高反应性环丙烷衍生物,它们的可及性受到严重限制。在此,我们报道了一种通过 Cp*Rh(III) 催化的环丙烯加氢酰氧基化作用实现多取代酰氧基环丙烷的高效立体选择性合成。该催化系统允许将各种廉价和市售的羧酸选择性地添加到环丙烯的双键中。该方案在非常温和的条件下进行,并显示出广泛的基团耐受性,提供未描述的酰氧基环丙烷,具有非常好的产量和独特的非对映选择性。此外,该反应可以在分子内对感兴趣的环丙基螺内酯进行。关键有机金属配合物的分离、NMR 监测研究、HMRS 实验和 DFT 计算揭示了重要的机理见解。
更新日期:2024-11-12
中文翻译:

环丙烯的 Rh 催化氢酰氧基化反应:酰氧基环丙烷的区域和非对映选择性合成
酰氧基环丙烷是生物和药物化学中必不可少的组成部分。然而,由于需要预合成高反应性环丙烷衍生物,它们的可及性受到严重限制。在此,我们报道了一种通过 Cp*Rh(III) 催化的环丙烯加氢酰氧基化作用实现多取代酰氧基环丙烷的高效立体选择性合成。该催化系统允许将各种廉价和市售的羧酸选择性地添加到环丙烯的双键中。该方案在非常温和的条件下进行,并显示出广泛的基团耐受性,提供未描述的酰氧基环丙烷,具有非常好的产量和独特的非对映选择性。此外,该反应可以在分子内对感兴趣的环丙基螺内酯进行。关键有机金属配合物的分离、NMR 监测研究、HMRS 实验和 DFT 计算揭示了重要的机理见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号