当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water activity: the key to unlocking high-voltage aqueous electrolytes?
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4ta06655c Yaroslav Zhigalenok, Saken Abdimomyn, Mikhael Levi, Netanel Shpigel, Margarita Ryabicheva, Maxim Lepikhin, Alina Galeyeva, Fyodor Malchik
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4ta06655c Yaroslav Zhigalenok, Saken Abdimomyn, Mikhael Levi, Netanel Shpigel, Margarita Ryabicheva, Maxim Lepikhin, Alina Galeyeva, Fyodor Malchik
|
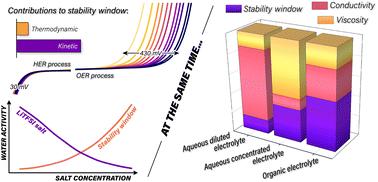
Aqueous electrolytes offer enhanced safety and environmental friendliness for next-generation energy storage systems, but a narrow electrochemical stability window limits their application. This study provides a comprehensive analysis of the relationship between water activity and the electrochemical stability window of aqueous electrolytes, critically examining current expansion strategies. Our investigation reveals that stability window expansion is primarily driven by kinetic factors rather than thermodynamic ones. We demonstrate that decreasing water activity predominantly affects the oxygen evolution reaction, with minimal impact on hydrogen evolution. This asymmetric effect is quantified through Tafel analysis, showing a significant decrease in exchange current density with reduced water activity. Notably, this study is the first to establish a direct correlation between water activity and the electrochemical stability window for aqueous electrolytes, providing fundamental insights into how water activity influences electrode reaction kinetics and overall system stability. We critically evaluate existing approaches to reducing water activity, including high-concentration electrolytes, water-in-salt systems, and hydrophobic ions. While these methods widen the electrochemical window, they lead to decreased ionic conductivity and increased viscosity. In “water-in-salt” electrolytes, conductivity drops to levels comparable to organic electrolytes while viscosity increases exponentially. This work challenges the focus on maximizing stability windows at the expense of other crucial properties. We argue for a balanced approach in aqueous electrolyte design, considering factors such as ionic mobility, salt solubility, viscosity, operational temperature range, and electrochemical stability.
中文翻译:

水分活度:解开高压水性电解质的关键?
水性电解质为下一代储能系统提供了更高的安全性和环境友好性,但较窄的电化学稳定性窗口限制了其应用。本研究全面分析了水活度与水性电解质电化学稳定性窗口之间的关系,批判性地研究了电流膨胀策略。我们的研究表明,稳定性窗口扩展主要由动力学因素而不是热力学因素驱动。我们证明,水活度降低主要影响析氧反应,对析氢的影响最小。这种不对称效应通过 Tafel 分析进行量化,显示交换电流密度显著降低,水活度降低。值得注意的是,这项研究首次建立了水活度与水性电解质电化学稳定性窗口之间的直接相关性,为水活度如何影响电极反应动力学和整体系统稳定性提供了基本见解。我们批判性地评估了减少水活度的现有方法,包括高浓度电解质、盐包水系统和疏水离子。虽然这些方法拓宽了电化学窗口,但它们会导致离子电导率降低和粘度增加。在“盐包水”电解质中,电导率下降到与有机电解质相当的水平,而粘度呈指数级增加。这项工作挑战了以牺牲其他关键特性为代价来最大化稳定性窗口的关注。 我们主张在水性电解质设计中采用平衡的方法,考虑离子迁移率、盐溶解度、粘度、操作温度范围和电化学稳定性等因素。
更新日期:2024-11-12
中文翻译:

水分活度:解开高压水性电解质的关键?
水性电解质为下一代储能系统提供了更高的安全性和环境友好性,但较窄的电化学稳定性窗口限制了其应用。本研究全面分析了水活度与水性电解质电化学稳定性窗口之间的关系,批判性地研究了电流膨胀策略。我们的研究表明,稳定性窗口扩展主要由动力学因素而不是热力学因素驱动。我们证明,水活度降低主要影响析氧反应,对析氢的影响最小。这种不对称效应通过 Tafel 分析进行量化,显示交换电流密度显著降低,水活度降低。值得注意的是,这项研究首次建立了水活度与水性电解质电化学稳定性窗口之间的直接相关性,为水活度如何影响电极反应动力学和整体系统稳定性提供了基本见解。我们批判性地评估了减少水活度的现有方法,包括高浓度电解质、盐包水系统和疏水离子。虽然这些方法拓宽了电化学窗口,但它们会导致离子电导率降低和粘度增加。在“盐包水”电解质中,电导率下降到与有机电解质相当的水平,而粘度呈指数级增加。这项工作挑战了以牺牲其他关键特性为代价来最大化稳定性窗口的关注。 我们主张在水性电解质设计中采用平衡的方法,考虑离子迁移率、盐溶解度、粘度、操作温度范围和电化学稳定性等因素。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号