当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evolving mineralogy and reactivity of hematite-coated sands during reduction of 4-chloronitrobenzene by Fe(II) in flow-through reactors
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4en00602j Celina M. Harris, Adel Soroush, Alanna M. Hildebrandt, Kamilah Y. Amen, Louis G. Corcoran, Joshua M. Feinberg, William A. Arnold, R. Lee Penn
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4en00602j Celina M. Harris, Adel Soroush, Alanna M. Hildebrandt, Kamilah Y. Amen, Louis G. Corcoran, Joshua M. Feinberg, William A. Arnold, R. Lee Penn
|
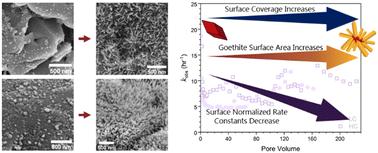
Naturally-occurring iron oxide nanoparticles provide reactive surfaces for the reduction of nitroaromatic compounds, which are common groundwater pollutants, by Fe(II). In many natural aquifer systems, iron oxide minerals continuously react with groundwater pollutants and other chemical species. To closely emulate field conditions, continuous flow columns packed with hematite-coated sands were used to study the reduction of 4-chloronitrobenzene (4-ClNB) by Fe(II) associated with the iron oxide. Columns were packed with sands coated with either a high or low mass loading of hematite nanoparticles (0.19 or 0.43 mg hematite per gram of sand after flushing). Following 36 hours of reaction (200–225 pore volumes), the total mass of iron oxide present in the columns increased, resulting from the concurrent Fe(III) oxidative mineral growth. The greatest increase was observed at the bottom of the column packed with the higher hematite mass loading sand. Acicular particles were observed on the post-reaction materials of both the high and low hematite loading sands. The acicular morphology is characteristic of goethite nanoparticles, and the presence of goethite was detected by low temperature magnetometry. Similar to results obtained under batch reactor conditions, goethite crystals heterogeneously nucleated on hematite as a result of the reductive degradation of 4-ClNB by Fe(II). Results tracking the rates of reductive degradation of the 4-ClNB and evolution of mineralogy demonstrate that reactivity is determined by the accessible reactive surface area, which evolves as goethite is deposited on hematite over time.
中文翻译:

在流通式反应器中 Fe(II) 还原 4-氯硝基苯过程中赤铁矿涂层砂的矿物学变化和反应性
天然存在的氧化铁纳米颗粒为 Fe(II) 还原硝基芳香族化合物(常见的地下水污染物)提供了反应性表面。在许多天然含水层系统中,氧化铁矿物不断与地下水污染物和其他化学物质发生反应。为了密切模拟现场条件,使用填充有赤铁矿涂层砂的连续流柱来研究与氧化铁相关的 Fe(II) 对 4-氯硝基苯 (4-ClNB) 的还原。塔填充有高质量或低质量负载的赤铁矿纳米颗粒(冲洗后每克沙子 0.19 或 0.43 毫克赤铁矿)。反应 36 小时(200–225 个孔体积)后,由于 Fe(III) 氧化矿物同时生长,色谱柱中存在的氧化铁总质量增加。在填充较高质量负载砂的塔底部观察到最大的增加。在高和低赤铁矿负载砂的反应后材料上都观察到针状颗粒。针状形态是针铁矿纳米颗粒的特征,通过低温磁力计检测针铁矿的存在。与在间歇式反应器条件下获得的结果类似,针铁矿晶体由于 Fe(II) 对 4-ClNB 的还原降解而在赤铁矿上异质成核。跟踪 4-ClNB 的还原降解速率和矿物学演变的结果表明,反应性取决于可接近的反应表面积,该表面积随着针铁矿沉积在赤铁矿上而演变。
更新日期:2024-11-12
中文翻译:

在流通式反应器中 Fe(II) 还原 4-氯硝基苯过程中赤铁矿涂层砂的矿物学变化和反应性
天然存在的氧化铁纳米颗粒为 Fe(II) 还原硝基芳香族化合物(常见的地下水污染物)提供了反应性表面。在许多天然含水层系统中,氧化铁矿物不断与地下水污染物和其他化学物质发生反应。为了密切模拟现场条件,使用填充有赤铁矿涂层砂的连续流柱来研究与氧化铁相关的 Fe(II) 对 4-氯硝基苯 (4-ClNB) 的还原。塔填充有高质量或低质量负载的赤铁矿纳米颗粒(冲洗后每克沙子 0.19 或 0.43 毫克赤铁矿)。反应 36 小时(200–225 个孔体积)后,由于 Fe(III) 氧化矿物同时生长,色谱柱中存在的氧化铁总质量增加。在填充较高质量负载砂的塔底部观察到最大的增加。在高和低赤铁矿负载砂的反应后材料上都观察到针状颗粒。针状形态是针铁矿纳米颗粒的特征,通过低温磁力计检测针铁矿的存在。与在间歇式反应器条件下获得的结果类似,针铁矿晶体由于 Fe(II) 对 4-ClNB 的还原降解而在赤铁矿上异质成核。跟踪 4-ClNB 的还原降解速率和矿物学演变的结果表明,反应性取决于可接近的反应表面积,该表面积随着针铁矿沉积在赤铁矿上而演变。






























 京公网安备 11010802027423号
京公网安备 11010802027423号