当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thieno[3,2-b]thiophene-based bridged BODIPY dimers: synthesis, electrochemistry, and one- and two-photon photophysical properties
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4dt02655a Zhiyong Chai, Tingting Gu, Annaelle Beau, Frédéric Bolze, Claude P. Gros, Xu Liang, Donghai Shi, Haijun Xu
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4dt02655a Zhiyong Chai, Tingting Gu, Annaelle Beau, Frédéric Bolze, Claude P. Gros, Xu Liang, Donghai Shi, Haijun Xu
|
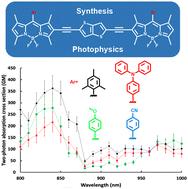
Four BODIPY dyes (6a–6d) with electron-donating or electron-withdrawing groups at the meso-position were synthesized by the Sonogashira coupling reaction of 2,5-diethynylthieno[3,2-b]thiophene with mono-iodo-BODIPY moieties. All compounds were fully characterized by 1H NMR and MALDI-TOF MS. Their photophysical and electrochemical properties were studied by UV-visible absorption spectroscopy, steady-state and time-resolved fluorescence spectroscopy, two-photon excitation spectroscopy and cyclic voltammetry. These conjugated dyes exhibit interesting photophysical properties such as a high molar extinction coefficient, large Stokes shift and high two-photon absorption cross section σ2.
中文翻译:

噻吩[3,2-b]噻吩基桥式 BODIPY 二聚体:合成、电化学以及单光子和双光子光物理性质
通过 2,5-二乙基噻吩[3,2-b]噻吩与一碘-BODIPY 部分的 Sonogashira 偶联反应合成了四种在介观位置具有供电子或吸电子基团的 BODIPY 染料 (6a–6d)。所有化合物均通过 1H NMR 和 MALDI-TOF MS 进行充分表征。通过紫外-可见吸收光谱、稳态和时间分辨荧光光谱、双光子激发光谱和循环伏安法研究了它们的光物理和电化学性质。这些偶联染料表现出有趣的光物理特性,例如高摩尔消光系数、大斯托克斯位移和高双光子吸收截面 σ 2。
更新日期:2024-11-12
中文翻译:

噻吩[3,2-b]噻吩基桥式 BODIPY 二聚体:合成、电化学以及单光子和双光子光物理性质
通过 2,5-二乙基噻吩[3,2-b]噻吩与一碘-BODIPY 部分的 Sonogashira 偶联反应合成了四种在介观位置具有供电子或吸电子基团的 BODIPY 染料 (6a–6d)。所有化合物均通过 1H NMR 和 MALDI-TOF MS 进行充分表征。通过紫外-可见吸收光谱、稳态和时间分辨荧光光谱、双光子激发光谱和循环伏安法研究了它们的光物理和电化学性质。这些偶联染料表现出有趣的光物理特性,例如高摩尔消光系数、大斯托克斯位移和高双光子吸收截面 σ 2。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号