当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-catalysed intramolecular C(sp3)–H amination of alkyl azides
Chemical Communications ( IF 4.3 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4cc04169k Kai Wu, Chi-Ming Che
Chemical Communications ( IF 4.3 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4cc04169k Kai Wu, Chi-Ming Che
|
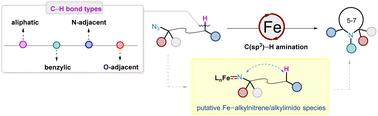
Iron-catalysed intramolecular C(sp3)–H amination of alkyl azides (N3R, R = alkyl) via the iron–alkylnitrene/alkylimido (Fe(NR)) intermediate, is an appealing synthetic approach for the synthesis of various N-heterocycles. This approach provides a direct atom-economy strategy for constructing C(sp3)–N bonds, with nitrogen gas as the only by-product and iron is a biocompatible, cheap, and earth-abundant metal. However, C(sp3)–H amination with alkyl azides is challenging because alkyl nitrenes readily undergo 1,2-hydride migration to imines. This article summarizes recent major advances in this field in terms of catalyst design, substrate scope expansion, stereoselectivity control, understanding of key reaction intermediates, and applications in the synthesis of complex natural products and pharmaceuticals.
中文翻译:

烷基叠氮化物的铁催化分子内 C(sp3)–H 胺化反应
铁催化的烷基叠氮化物(N3R,R = 烷基)的分子内 C(sp3)-H 胺化反应通过铁-烷基腈/烷基酰亚胺 (Fe(NR)) 中间体,是一种吸引人的合成方法,用于合成各种 N-杂环。这种方法为构建 C(sp3)-N 键提供了一种直接的原子经济策略,氮气是唯一的副产品,铁是一种生物相容性、廉价且地球上丰富的金属。然而,用烷基叠氮化物进行 C(sp3)–H 胺化反应具有挑战性,因为烷基硝酸很容易发生 1,2-氢化物迁移到亚胺上。本文总结了该领域在催化剂设计、底物范围扩展、立体选择性控制、关键反应中间体的理解以及在复杂天然产物和药物合成中的应用方面的最新重大进展。
更新日期:2024-11-12
中文翻译:

烷基叠氮化物的铁催化分子内 C(sp3)–H 胺化反应
铁催化的烷基叠氮化物(N3R,R = 烷基)的分子内 C(sp3)-H 胺化反应通过铁-烷基腈/烷基酰亚胺 (Fe(NR)) 中间体,是一种吸引人的合成方法,用于合成各种 N-杂环。这种方法为构建 C(sp3)-N 键提供了一种直接的原子经济策略,氮气是唯一的副产品,铁是一种生物相容性、廉价且地球上丰富的金属。然而,用烷基叠氮化物进行 C(sp3)–H 胺化反应具有挑战性,因为烷基硝酸很容易发生 1,2-氢化物迁移到亚胺上。本文总结了该领域在催化剂设计、底物范围扩展、立体选择性控制、关键反应中间体的理解以及在复杂天然产物和药物合成中的应用方面的最新重大进展。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号