当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-catalyzed stereospecific reductive cross-coupling of vinyl chlorosilanes with axially chiral biaryl electrophiles
Chemical Communications ( IF 4.3 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4cc04293j Tiantian Yin, Shiyuan Sui, Shuqi Li, Junbiao Chang, Dachang Bai
Chemical Communications ( IF 4.3 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4cc04293j Tiantian Yin, Shiyuan Sui, Shuqi Li, Junbiao Chang, Dachang Bai
|
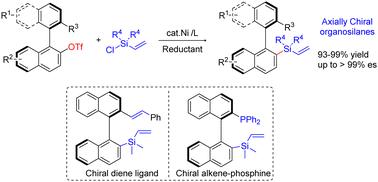
Enantioenriched organosilanes are important chiral molecules in materials science and organic synthesis. The synthesis of axially chiral organosilanes is particularly significant in terms of applications. Herein, we report a Ni-catalyzed reductive cross-electrophile coupling of vinyl chlorosilanes with sterically hindered chiral biaryl electrophiles for the synthesis of atropisomeric biaryl organosilanes. Various enantioenriched axially chiral vinylsilanes are accessible in high efficiency under mild conditions. The synthetic transformations and applications of new chiral silicon-containing alkene ligands are demonstrated.
中文翻译:

乙烯基氯硅烷与轴向手性联芳基亲电试剂的镍催化立体特异性还原偶联
富含对映体的有机硅烷是材料科学和有机合成中重要的手性分子。轴向手性有机硅烷的合成在应用方面尤为重要。在此,我们报道了乙烯基氯硅烷与空间受阻手性联芳基亲电试剂的 Ni 催化还原交叉亲电偶联,用于合成偏斜异构联芳基有机硅烷。在温和的条件下,可以高效地获得各种富含对映体的轴向手性乙烯基硅烷。展示了新的手性含硅烯烃配体的合成转化和应用。
更新日期:2024-11-12
中文翻译:

乙烯基氯硅烷与轴向手性联芳基亲电试剂的镍催化立体特异性还原偶联
富含对映体的有机硅烷是材料科学和有机合成中重要的手性分子。轴向手性有机硅烷的合成在应用方面尤为重要。在此,我们报道了乙烯基氯硅烷与空间受阻手性联芳基亲电试剂的 Ni 催化还原交叉亲电偶联,用于合成偏斜异构联芳基有机硅烷。在温和的条件下,可以高效地获得各种富含对映体的轴向手性乙烯基硅烷。展示了新的手性含硅烯烃配体的合成转化和应用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号