当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural, Solvent, and Temperature Effects on Protein Junction Conductance
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.jpclett.4c02230 Gowtham Nirmal Jonnalagadda, Xiaojing Wu, Lukáš Hronek, Zdenek Futera
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.jpclett.4c02230 Gowtham Nirmal Jonnalagadda, Xiaojing Wu, Lukáš Hronek, Zdenek Futera
|
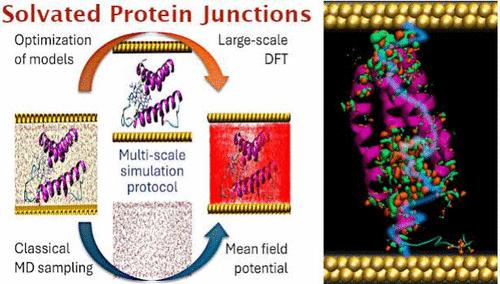
Cytochrome b562 is a small redox-active heme protein that has served as an important model system for understanding biological electron transfer processes. Here, we present a comprehensive theoretical study of electron transport mechanisms in protein–metal junctions incorporating cytochrome b562 using a multi-scale computational approach. Employing molecular dynamics (MD) simulations, we generated junction geometries for both vacuum-dried and solvated conditions, with the protein covalently bound to gold contacts in various configurations. Coherent tunneling, described by the Landauer–Buttiker formalism within the density functional theory (DFT) framework, is compared to the incoherent hopping charge transport mechanism captured by the semi-classical Marcus theory. The tunneling was identified as the dominant mechanism explaining the experimental data measured on the cytochrome b562 junctions, exhibiting exponential yet very shallow distance dependence. While the structural orientations and protein contacts with the electrodes influence the junction conductance significantly, the solvation effects are relatively small, affecting the electronic properties mostly via the adsorption arrangement. On the other hand, the considerable temperature dependence of the conductance was found strong only for hopping, while the tunneling current magnitudes remain practically unaffected and are a good indicator of the coherent mechanism in this case.
中文翻译:

结构、溶剂和温度对蛋白质连接电导率的影响
细胞色素 b562 是一种小的氧化还原活性血红素蛋白,是理解生物电子转移过程的重要模型系统。在这里,我们使用多尺度计算方法对掺入细胞色素 b562 的蛋白质-金属连接中的电子传递机制进行了全面的理论研究。采用分子动力学 (MD) 模拟,我们生成了真空干燥和溶剂化条件的液络部几何形状,其中蛋白质以各种构型共价结合到金触点上。将密度泛函理论 (DFT) 框架内 Landauer-Buttiker 形式主义描述的相干隧穿与半经典 Marcus 理论捕获的不相干跳跃电荷传输机制进行了比较。隧穿被确定为解释在细胞色素 b562 连接上测量的实验数据的主要机制,表现出指数级但非常浅的距离依赖性。虽然结构取向和蛋白质与电极的接触对结电导有显著影响,但溶剂化效应相对较小,主要通过吸附排列影响电子性能。另一方面,仅对于跳跃,发现电导的相当大的温度依赖性很强,而隧穿电流幅度几乎不受影响,在这种情况下是相干机制的良好指标。
更新日期:2024-11-12
中文翻译:

结构、溶剂和温度对蛋白质连接电导率的影响
细胞色素 b562 是一种小的氧化还原活性血红素蛋白,是理解生物电子转移过程的重要模型系统。在这里,我们使用多尺度计算方法对掺入细胞色素 b562 的蛋白质-金属连接中的电子传递机制进行了全面的理论研究。采用分子动力学 (MD) 模拟,我们生成了真空干燥和溶剂化条件的液络部几何形状,其中蛋白质以各种构型共价结合到金触点上。将密度泛函理论 (DFT) 框架内 Landauer-Buttiker 形式主义描述的相干隧穿与半经典 Marcus 理论捕获的不相干跳跃电荷传输机制进行了比较。隧穿被确定为解释在细胞色素 b562 连接上测量的实验数据的主要机制,表现出指数级但非常浅的距离依赖性。虽然结构取向和蛋白质与电极的接触对结电导有显著影响,但溶剂化效应相对较小,主要通过吸附排列影响电子性能。另一方面,仅对于跳跃,发现电导的相当大的温度依赖性很强,而隧穿电流幅度几乎不受影响,在这种情况下是相干机制的良好指标。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号