当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Process Development for the Manufacture of a Topical Pan-Trk Inhibitor Incorporating Decarboxylative sp2–sp3 Cross-Coupling
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.oprd.4c00325 Michael S. Ashwood, Edward I. Balmond, David Fengas, Jane McGuffog, Jonathan Moore, Nicola M. Robas, Neil G. Stevenson, Lisa Wise
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-12 , DOI: 10.1021/acs.oprd.4c00325 Michael S. Ashwood, Edward I. Balmond, David Fengas, Jane McGuffog, Jonathan Moore, Nicola M. Robas, Neil G. Stevenson, Lisa Wise
|
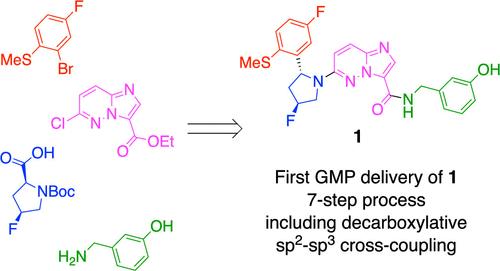
The development of a synthetic route toward topical pan-Trk inhibitor 1 is described as an eight-stage synthesis from available starting materials. Process improvements include the development of a decarboxylative sp2–sp3 cross-coupling which had not previously been demonstrated on scale. Parameters were explored, balancing the safety aspects with conversion and selectivity, scaling up in a stepwise fashion to multiple successful 0.7 kg batches. The cross-coupling showed high diastereoselectivity, with the opposite diastereomer not observed in the crude 19F NMR. Selectivity was further improved by crystallizing the downstream pyrrolidine salt after Boc deprotection, to give a diastereomer ratio of 99.5:0.5 by UPLC. This route has been reproducibly demonstrated in two GMP campaigns delivering API on kilogram scale, in >98% area purity by HPLC. The route design, solid-form screening, process research, and manufacture have enabled crucial first-in-human (FIH) clinical studies, through focus on speed of delivery.
中文翻译:

生产掺入脱羧 sp2-sp3 交叉偶联的局部 Pan-Trk 抑制剂的工艺开发
针对局部 pan-Trk 抑制剂 1 的合成路线的开发被描述为从可用起始材料进行八阶段合成。工艺改进包括开发脱羧 sp2-sp 3 交叉偶联,这在以前从未进行过大规模验证。探索了参数,平衡了安全性与转化率和选择性,逐步扩大到多个成功的 0.7 kg 批次。交叉偶联显示出高非对映选择性,在粗制 19F NMR 中未观察到相反的非对映异构体。通过在 Boc 脱保护后结晶下游吡咯烷盐进一步提高选择性,通过 UPLC 得到 99.5:0.5 的非对映异构体比。该路线已在两次 GMP 活动中得到可重复的证明,通过 HPLC 以 >98% 的峰面积纯度提供千克级 API。通过关注交付速度,路线设计、实体形式筛选、工艺研究和制造使关键的首次人体 (FIH) 临床研究成为可能。
更新日期:2024-11-12
中文翻译:

生产掺入脱羧 sp2-sp3 交叉偶联的局部 Pan-Trk 抑制剂的工艺开发
针对局部 pan-Trk 抑制剂 1 的合成路线的开发被描述为从可用起始材料进行八阶段合成。工艺改进包括开发脱羧 sp2-sp 3 交叉偶联,这在以前从未进行过大规模验证。探索了参数,平衡了安全性与转化率和选择性,逐步扩大到多个成功的 0.7 kg 批次。交叉偶联显示出高非对映选择性,在粗制 19F NMR 中未观察到相反的非对映异构体。通过在 Boc 脱保护后结晶下游吡咯烷盐进一步提高选择性,通过 UPLC 得到 99.5:0.5 的非对映异构体比。该路线已在两次 GMP 活动中得到可重复的证明,通过 HPLC 以 >98% 的峰面积纯度提供千克级 API。通过关注交付速度,路线设计、实体形式筛选、工艺研究和制造使关键的首次人体 (FIH) 临床研究成为可能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号