当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Chemoenzymatic Cascade for the Formal Enantioselective Hydroxylation and Amination of Benzylic C–H Bonds
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acscatal.4c03161 Yuqing Zhang, Chen Huang, Weixi Kong, Liya Zhou, Jing Gao, Frank Hollmann, Yunting Liu, Yanjun Jiang
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acscatal.4c03161 Yuqing Zhang, Chen Huang, Weixi Kong, Liya Zhou, Jing Gao, Frank Hollmann, Yunting Liu, Yanjun Jiang
|
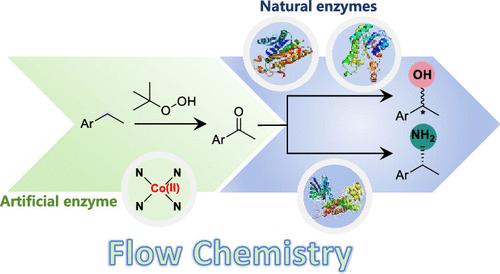
We report the synthesis and characterization of an artificial peroxygenase (CoN4SA-POase) with CoN4 active sites by supporting single-atom cobalt on polymeric carbon nitrogen, which exhibits high activity, selectivity, stability, and reusability in the oxidation of aromatic alkanes to ketones. Density functional theory calculations reveal a different catalytic mechanism for the artificial peroxygenase from that of natural peroxygenases. In addition, continuous-flow systems are employed to combine CoN4SA-POase with enantiocomplementary ketoreductases as well as an amine dehydrogenase, enabling the enantioselective synthesis of chiral alcohols and amines from hydrocarbons with significantly improved productivity. This work, emulating nature and beyond nature, provides a promising design concept for heme enzyme-based transformations.
中文翻译:

用于苄基 C-H 键的正式对映选择性羟基化和胺化的化学酶级联反应
我们报道了通过在聚合物碳氮上支持单原子钴来合成和表征具有 CoN4 活性位点的人工过加氧酶 (CoN4SA-POase),其在芳香族烷烃氧化为酮方面表现出高活性、选择性、稳定性和可重用性。密度泛函理论计算揭示了人工过加氧酶与天然过加氧酶不同的催化机制。此外,采用连续流系统将 CoN4SA-POase 与对映体互补酮还原酶以及胺脱氢酶相结合,能够从碳氢化合物中对映选择性合成手性醇和胺,显著提高生产率。这项工作模拟自然和超越自然,为基于血红素酶的转化提供了一个有前途的设计概念。
更新日期:2024-11-12
中文翻译:

用于苄基 C-H 键的正式对映选择性羟基化和胺化的化学酶级联反应
我们报道了通过在聚合物碳氮上支持单原子钴来合成和表征具有 CoN4 活性位点的人工过加氧酶 (CoN4SA-POase),其在芳香族烷烃氧化为酮方面表现出高活性、选择性、稳定性和可重用性。密度泛函理论计算揭示了人工过加氧酶与天然过加氧酶不同的催化机制。此外,采用连续流系统将 CoN4SA-POase 与对映体互补酮还原酶以及胺脱氢酶相结合,能够从碳氢化合物中对映选择性合成手性醇和胺,显著提高生产率。这项工作模拟自然和超越自然,为基于血红素酶的转化提供了一个有前途的设计概念。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号