当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric C–H Activation/Cyclization of Sulfoximines with Sulfoxonium Ylides by a Chiral η6-Benzene Ruthenium(II) Catalyst
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acscatal.4c04798 Huan Liu, Ji-Jun Jiang, Jun Wang
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-12 , DOI: 10.1021/acscatal.4c04798 Huan Liu, Ji-Jun Jiang, Jun Wang
|
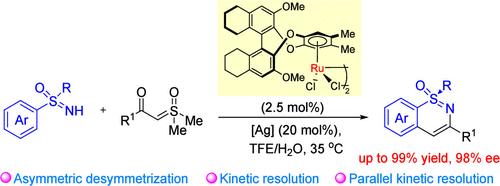
Chiral η6-benzene ruthenium(II) (BenRuII)-catalyzed asymmetric C–H activations are challenging and rarely seen in the literature. Herein, the asymmetric C–H activation/cyclization of sulfoximines with sulfoxonium ylides catalyzed by the chiral BenRuII catalyst derived from (S)-H8–BINOL is described. It provides efficient access to various sulfur-chiral 1,2-benzothiazine 1-oxides in high yields with high enantioselectivities (up to 99% yield and 98% ee). Kinetic resolution of racemic sulfoximines was also feasible. The reaction mechanism was studied by the tool of H/D exchange and the kinetic isotope effect. The metallacycle revealing the origin of chiral induction was prepared, characterized, and proved effective for the model reaction. This work demonstrates the great potential of chiral BenRuII catalysts for asymmetric C–H activation.
中文翻译:

通过手性 η6-苯钌 (II) 催化剂催化不对称 C-H 活化/环化亚砜与烯基磺铵
手性η6-苯钌 (II) (BenRuII) 催化的不对称 C-H 活化具有挑战性,在文献中很少见。本文描述了由 (S)-H 8-BINOL 衍生的手性 BenRuII 催化剂催化的亚砜胺与磺酰铵的不对称 C-H 活化/环化。它以高产率和高对映选择性(高达 99% 的收率和 98% 的 ee)高效获取各种硫手性 1,2-苯并噻嗪 1-氧化物。外消旋砜虫胺的动力学分离也是可行的。利用 H/D 交换工具和动力学同位素效应研究了反应机理。制备、表征并证明对模型反应有效的揭示手性诱导起源的金属循环。这项工作证明了手性 BenRuII 催化剂在不对称 C-H 活化方面的巨大潜力。
更新日期:2024-11-12
中文翻译:

通过手性 η6-苯钌 (II) 催化剂催化不对称 C-H 活化/环化亚砜与烯基磺铵
手性η6-苯钌 (II) (BenRuII) 催化的不对称 C-H 活化具有挑战性,在文献中很少见。本文描述了由 (S)-H 8-BINOL 衍生的手性 BenRuII 催化剂催化的亚砜胺与磺酰铵的不对称 C-H 活化/环化。它以高产率和高对映选择性(高达 99% 的收率和 98% 的 ee)高效获取各种硫手性 1,2-苯并噻嗪 1-氧化物。外消旋砜虫胺的动力学分离也是可行的。利用 H/D 交换工具和动力学同位素效应研究了反应机理。制备、表征并证明对模型反应有效的揭示手性诱导起源的金属循环。这项工作证明了手性 BenRuII 催化剂在不对称 C-H 活化方面的巨大潜力。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号