Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three-Dimensional Semiconductor Network as Regulators of Energy Metabolism Drives Angiogenesis in Bone Regeneration
ACS Nano ( IF 15.8 ) Pub Date : 2024-11-12 , DOI: 10.1021/acsnano.4c09971 Youzhun Fan, Jiwei Sun, Wenjie Fan, Xianwei Zhong, Zhaoyi Yin, Bin Su, Jing Yao, Xinyu Hong, Jinxia Zhai, Zhengao Wang, Haoyan Chen, Fengyuan Guo, Xiufang Wen, Chengyun Ning, Lili Chen, Peng Yu
ACS Nano ( IF 15.8 ) Pub Date : 2024-11-12 , DOI: 10.1021/acsnano.4c09971 Youzhun Fan, Jiwei Sun, Wenjie Fan, Xianwei Zhong, Zhaoyi Yin, Bin Su, Jing Yao, Xinyu Hong, Jinxia Zhai, Zhengao Wang, Haoyan Chen, Fengyuan Guo, Xiufang Wen, Chengyun Ning, Lili Chen, Peng Yu
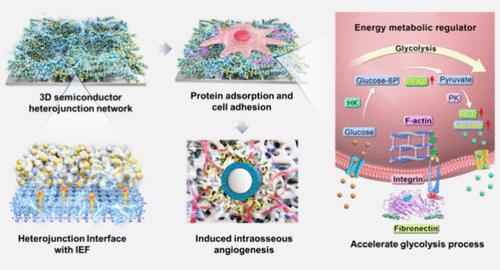
|
Insufficient vascularization is a primary cause of bone implantation failure. The management of energy metabolism is crucial for the achievement of vascularized osseointegration. In light of the bone semiconductor property and the electric property of semiconductor heterojunctions, a three-dimensional semiconductor heterojunction network (3D-NTBH) implant has been devised with the objective of regulating cellular energy metabolism, thereby driving angiogenesis for bone regeneration. The three-dimensional heterojunction interfaces facilitate electron transfer and establish internal electric fields at the nanoscale interfaces. The 3D-NTBH was found to noticeably accelerate glycolysis in endothelial cells, thereby rapidly providing energy to support cellular metabolic activities and ultimately driving angiogenesis within the bone tissue. Molecular dynamic simulations have demonstrated that the 3D-NTBH facilitates the exposure of fibronectin’s Arg-Gly-Asp peptide binding site, thereby regulating the glycolysis of endothelial cells. Further evidence suggests that 3D-NTBH promotes early vascular network reconstruction and bone regeneration in vivo. The findings of this research offer a promising research perspective for the design of vascularizing implants.
中文翻译:

三维半导体网络作为能量代谢的调节因子驱动骨再生中的血管生成
血管形成不足是骨植入失败的主要原因。能量代谢的管理对于实现血管化骨整合至关重要。鉴于骨半导体特性和半导体异质结的电特性,已经设计了一种三维半导体异质结网络 (3D-NTBH) 植入物,目的是调节细胞能量代谢,从而驱动血管生成以实现骨再生。三维异质结界面促进了电子转移并在纳米级界面处建立了内部电场。发现 3D-NTBH 显着加速内皮细胞中的糖酵解,从而迅速提供能量以支持细胞代谢活动,并最终驱动骨组织内的血管生成。分子动力学模拟表明,3D-NTBH 有助于纤连蛋白的 Arg-Gly-Asp 肽结合位点的暴露,从而调节内皮细胞的糖酵解。进一步的证据表明,3D-NTBH 促进体内早期血管网络重建和骨再生。这项研究的结果为血管化植入物的设计提供了有前途的研究前景。
更新日期:2024-11-12
中文翻译:

三维半导体网络作为能量代谢的调节因子驱动骨再生中的血管生成
血管形成不足是骨植入失败的主要原因。能量代谢的管理对于实现血管化骨整合至关重要。鉴于骨半导体特性和半导体异质结的电特性,已经设计了一种三维半导体异质结网络 (3D-NTBH) 植入物,目的是调节细胞能量代谢,从而驱动血管生成以实现骨再生。三维异质结界面促进了电子转移并在纳米级界面处建立了内部电场。发现 3D-NTBH 显着加速内皮细胞中的糖酵解,从而迅速提供能量以支持细胞代谢活动,并最终驱动骨组织内的血管生成。分子动力学模拟表明,3D-NTBH 有助于纤连蛋白的 Arg-Gly-Asp 肽结合位点的暴露,从而调节内皮细胞的糖酵解。进一步的证据表明,3D-NTBH 促进体内早期血管网络重建和骨再生。这项研究的结果为血管化植入物的设计提供了有前途的研究前景。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号