Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Opposing roles for AMPK in regulating distinct mitophagy pathways
Molecular Cell ( IF 14.5 ) Pub Date : 2024-11-11 , DOI: 10.1016/j.molcel.2024.10.025 Marianna Longo, Aniketh Bishnu, Pierpaolo Risiglione, Lambert Montava-Garriga, Joyceline Cuenco, Kei Sakamoto, Carol MacKintosh, Ian G. Ganley
Molecular Cell ( IF 14.5 ) Pub Date : 2024-11-11 , DOI: 10.1016/j.molcel.2024.10.025 Marianna Longo, Aniketh Bishnu, Pierpaolo Risiglione, Lambert Montava-Garriga, Joyceline Cuenco, Kei Sakamoto, Carol MacKintosh, Ian G. Ganley
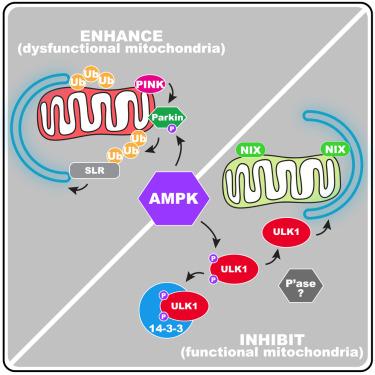
|
Mitophagy degrades damaged mitochondria, but we show here that it can also target functional mitochondria. This latter scenario occurs during programmed mitophagy and involves the mitophagy receptors NIX and BNIP3. Although AMP-activated protein kinase (AMPK), the energy-sensing protein kinase, can influence damaged-induced mitophagy, its role in programmed mitophagy is unclear. We found that AMPK directly inhibits NIX-dependent mitophagy by triggering 14-3-3-mediated sequestration of ULK1, via ULK1 phosphorylation at two sites: Ser556 and an additional identified site, Ser694. By contrast, AMPK activation increases Parkin phosphorylation and enhances the rate of depolarization-induced mitophagy, independently of ULK1. We show that this happens both in cultured cells and tissues in vivo, using the mito-QC mouse model. Our work unveils a mechanism whereby AMPK activation downregulates mitophagy of functional mitochondria but enhances that of dysfunctional/damaged ones.
中文翻译:

AMPK 在调节不同线粒体自噬途径中的作用相反
线粒体自噬降解受损的线粒体,但我们在这里表明它也可以靶向功能性线粒体。后一种情况发生在程序性线粒体自噬期间,涉及线粒体自噬受体 NIX 和 BNIP3。尽管 AMP 活化蛋白激酶 (AMPK) 是一种能量感应蛋白激酶,可以影响受损诱导的线粒体自噬,但其在程序性线粒体自噬中的作用尚不清楚。我们发现 AMPK 通过在两个位点的 ULK1 磷酸化触发 14-3-3 介导的 ULK1 隔离,直接抑制 NIX 依赖性线粒体自噬:Ser556 和另一个已确定的位点 Ser694。相比之下,AMPK 激活会增加 Parkin 磷酸化并增强去极化诱导的线粒体自噬速率,这与 ULK1 无关。我们使用 mito-QC 小鼠模型表明,这发生在培养细胞和体内组织中 。我们的工作揭示了一种机制,即 AMPK 激活下调功能性线粒体的线粒体自噬,但增强功能失调/受损线粒体的线粒体自噬。
更新日期:2024-11-11
中文翻译:

AMPK 在调节不同线粒体自噬途径中的作用相反
线粒体自噬降解受损的线粒体,但我们在这里表明它也可以靶向功能性线粒体。后一种情况发生在程序性线粒体自噬期间,涉及线粒体自噬受体 NIX 和 BNIP3。尽管 AMP 活化蛋白激酶 (AMPK) 是一种能量感应蛋白激酶,可以影响受损诱导的线粒体自噬,但其在程序性线粒体自噬中的作用尚不清楚。我们发现 AMPK 通过在两个位点的 ULK1 磷酸化触发 14-3-3 介导的 ULK1 隔离,直接抑制 NIX 依赖性线粒体自噬:Ser556 和另一个已确定的位点 Ser694。相比之下,AMPK 激活会增加 Parkin 磷酸化并增强去极化诱导的线粒体自噬速率,这与 ULK1 无关。我们使用 mito-QC 小鼠模型表明,这发生在培养细胞和体内组织中 。我们的工作揭示了一种机制,即 AMPK 激活下调功能性线粒体的线粒体自噬,但增强功能失调/受损线粒体的线粒体自噬。






























 京公网安备 11010802027423号
京公网安备 11010802027423号