当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulated GATA1 expression as a universal gene therapy for Diamond-Blackfan anemia
Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-11-11 , DOI: 10.1016/j.stem.2024.10.012 Richard A. Voit, Xiaotian Liao, Alexis Caulier, Mateusz Antoszewski, Blake Cohen, Myriam Armant, Henry Y. Lu, Travis J. Fleming, Elena Kamal, Lara Wahlster, Aoife M. Roche, John K. Everett, Angelina Petrichenko, Mei-Mei Huang, William Clarke, Kasiani C. Myers, Craig Forester, Antonio Perez-Atayde, Frederic D. Bushman, Danilo Pellin, Vijay G. Sankaran
Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-11-11 , DOI: 10.1016/j.stem.2024.10.012 Richard A. Voit, Xiaotian Liao, Alexis Caulier, Mateusz Antoszewski, Blake Cohen, Myriam Armant, Henry Y. Lu, Travis J. Fleming, Elena Kamal, Lara Wahlster, Aoife M. Roche, John K. Everett, Angelina Petrichenko, Mei-Mei Huang, William Clarke, Kasiani C. Myers, Craig Forester, Antonio Perez-Atayde, Frederic D. Bushman, Danilo Pellin, Vijay G. Sankaran
|
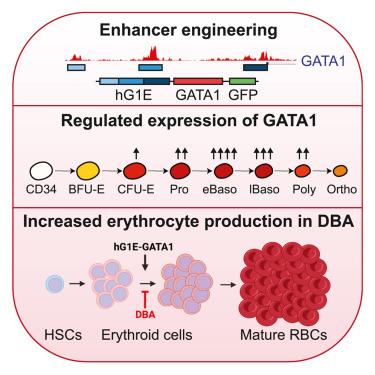
Gene therapy using hematopoietic stem and progenitor cells is altering the therapeutic landscape for patients with hematologic, immunologic, and metabolic disorders but has not yet been successfully developed for individuals with the bone marrow failure syndrome Diamond-Blackfan anemia (DBA). More than 30 mutations cause DBA through impaired ribosome function and lead to inefficient translation of the erythroid master regulator GATA1, providing a potential avenue for therapeutic intervention applicable to all patients with DBA, irrespective of the underlying genotype. Here, we report the development of a clinical-grade lentiviral gene therapy that achieves erythroid lineage-restricted expression of GATA1. We show that this vector is capable of augmenting erythropoiesis in DBA models and diverse patient samples without impacting hematopoietic stem cell function or demonstrating any signs of premalignant clonal expansion. These preclinical safety and efficacy data provide strong support for the first-in-human universal gene therapy trial for DBA through regulated GATA1 expression.
中文翻译:

调节 GATA1 表达作为 Diamond-Blackfan 贫血的通用基因疗法
使用造血干细胞和祖细胞的基因疗法正在改变血液学、免疫学和代谢紊乱患者的治疗前景,但尚未成功开发用于骨髓衰竭综合征 Diamond-Blackfan 贫血 (DBA) 患者。超过 30 种突变通过核糖体功能受损导致 DBA,并导致红系主调节因子 GATA1 的翻译效率低下,为适用于所有 DBA 患者的治疗干预提供了一条潜在的途径,无论潜在的基因型如何。在这里,我们报道了一种临床级慢病毒基因疗法的开发,该疗法实现了 GATA1 的红系限制性表达。我们表明,该载体能够在 DBA 模型和不同患者样本中增加红细胞生成,而不会影响造血干细胞功能或表现出任何癌前克隆扩增的迹象。这些临床前安全性和有效性数据为通过调节 GATA1 表达的 DBA 首次人体通用基因治疗试验提供了有力支持。
更新日期:2024-11-12
中文翻译:

调节 GATA1 表达作为 Diamond-Blackfan 贫血的通用基因疗法
使用造血干细胞和祖细胞的基因疗法正在改变血液学、免疫学和代谢紊乱患者的治疗前景,但尚未成功开发用于骨髓衰竭综合征 Diamond-Blackfan 贫血 (DBA) 患者。超过 30 种突变通过核糖体功能受损导致 DBA,并导致红系主调节因子 GATA1 的翻译效率低下,为适用于所有 DBA 患者的治疗干预提供了一条潜在的途径,无论潜在的基因型如何。在这里,我们报道了一种临床级慢病毒基因疗法的开发,该疗法实现了 GATA1 的红系限制性表达。我们表明,该载体能够在 DBA 模型和不同患者样本中增加红细胞生成,而不会影响造血干细胞功能或表现出任何癌前克隆扩增的迹象。这些临床前安全性和有效性数据为通过调节 GATA1 表达的 DBA 首次人体通用基因治疗试验提供了有力支持。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号