当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Shifting hydrogenation pathway via electronic activation for efficient nitrate electroreduction to ammonia in sewages
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-11-12 , DOI: 10.1016/j.checat.2024.101182 Wenye Zhong, Xuepeng Xiang, Peiyan Chen, Jiayu Su, Zhiheng Gong, Xueming Liu, Shijun Zhao, Nian Zhang, Chunhua Feng, Zhibin Zhang, Yan Chen, Zhang Lin
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-11-12 , DOI: 10.1016/j.checat.2024.101182 Wenye Zhong, Xuepeng Xiang, Peiyan Chen, Jiayu Su, Zhiheng Gong, Xueming Liu, Shijun Zhao, Nian Zhang, Chunhua Feng, Zhibin Zhang, Yan Chen, Zhang Lin
|
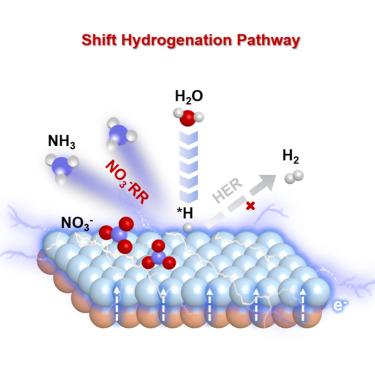
Electrochemical hydrogenation reactions have attracted worldwide attention as a sustainable alternative to thermo-catalytic hydrogenations. Nevertheless, the Faradaic efficiency, in many cases, is limited by the competing side reaction of hydrogen evolution. In this work, we demonstrate that the hydrogenation pathway can be effectively modulated by electronic activation near the interface. In a heterostructure consisting of a Cu foam matrix and Co3 O4 decoration layer (Co@Cu), the surface Co is effectively activated by electrons transferring from underneath Cu, leading to strongly promoted reactant adsorption and weakened Co-H bonding. Consequently, the hydrogenation pathway on the Co site shifts from H-H coupling to nitrate reduction, resulting in an outstanding nitrate reduction reaction (NO3 − RR) Faradaic efficiency of 97.67%. A hybrid reactor combining electroreduction and membrane separation is further constructed to realize an NH3 recovery rate as high as 857.1 g-N m−2 d−1 from actual sewage. The results can be generalized for other electrochemical hydrogenation reactions for energy and environment applications.
中文翻译:

通过电子活化改变加氢途径,在污水中将硝酸盐高效电还原为氨
电化学加氢反应作为热催化加氢的可持续替代品引起了全世界的关注。然而,在许多情况下,法拉第效率受到析氢的竞争性副反应的限制。在这项工作中,我们证明了氢化途径可以通过界面附近的电子活化进行有效调节。在由泡沫铜基体和 Co3O4 装饰层 (Co@Cu) 组成的异质结构中,表面 Co 被从 Cu 下方转移的电子有效激活,导致强烈促进反应物吸附和 Co-H 键合减弱。因此,Co 位点的氢化途径从 H-H 偶联转变为硝酸盐还原,从而产生了 97.67% 的出色硝酸盐还原反应 (NO3−RR) 法拉第效率。进一步构建了电还原和膜分离相结合的混合反应器,以实现从实际污水中回收高达 857.1 g-N m-2 d-1 的 NH3 回收率。结果可以推广到能源和环境应用的其他电化学加氢反应中。
更新日期:2024-11-12
中文翻译:

通过电子活化改变加氢途径,在污水中将硝酸盐高效电还原为氨
电化学加氢反应作为热催化加氢的可持续替代品引起了全世界的关注。然而,在许多情况下,法拉第效率受到析氢的竞争性副反应的限制。在这项工作中,我们证明了氢化途径可以通过界面附近的电子活化进行有效调节。在由泡沫铜基体和 Co3O4 装饰层 (Co@Cu) 组成的异质结构中,表面 Co 被从 Cu 下方转移的电子有效激活,导致强烈促进反应物吸附和 Co-H 键合减弱。因此,Co 位点的氢化途径从 H-H 偶联转变为硝酸盐还原,从而产生了 97.67% 的出色硝酸盐还原反应 (NO3−RR) 法拉第效率。进一步构建了电还原和膜分离相结合的混合反应器,以实现从实际污水中回收高达 857.1 g-N m-2 d-1 的 NH3 回收率。结果可以推广到能源和环境应用的其他电化学加氢反应中。































 京公网安备 11010802027423号
京公网安备 11010802027423号