Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decreased lipidated ApoE-receptor interactions confer protection against pathogenicity of ApoE and its lipid cargoes in lysosomes
Cell ( IF 45.5 ) Pub Date : 2024-11-11 , DOI: 10.1016/j.cell.2024.10.027 Jing L. Guo, Dylan Braun, Gabriel A. Fitzgerald, Yun-Ting Hsieh, Lionel Rougé, Alexandra Litvinchuk, Micah Steffek, Nicholas E. Propson, Catherine M. Heffner, Claire Discenza, Suk Ji Han, Anil Rana, Lukas L. Skuja, Bi Qi Lin, Elizabeth W. Sun, Sonnet S. Davis, Srijana Balasundar, Isabel Becerra, Jason C. Dugas, Connie Ha, Gilbert Di Paolo
Cell ( IF 45.5 ) Pub Date : 2024-11-11 , DOI: 10.1016/j.cell.2024.10.027 Jing L. Guo, Dylan Braun, Gabriel A. Fitzgerald, Yun-Ting Hsieh, Lionel Rougé, Alexandra Litvinchuk, Micah Steffek, Nicholas E. Propson, Catherine M. Heffner, Claire Discenza, Suk Ji Han, Anil Rana, Lukas L. Skuja, Bi Qi Lin, Elizabeth W. Sun, Sonnet S. Davis, Srijana Balasundar, Isabel Becerra, Jason C. Dugas, Connie Ha, Gilbert Di Paolo
|
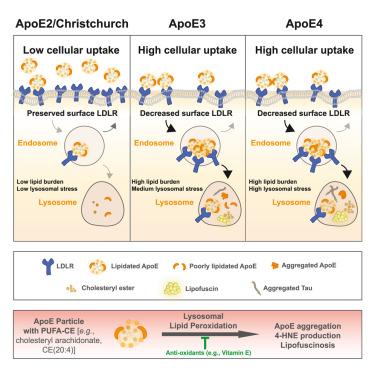
While apolipoprotein E (APOE) is the strongest genetic modifier for late-onset Alzheimer’s disease (LOAD), the molecular mechanisms underlying isoform-dependent risk and the relevance of ApoE-associated lipids remain elusive. Here, we report that impaired low-density lipoprotein (LDL) receptor (LDLR) binding of lipidated ApoE2 (lipApoE2) avoids LDLR recycling defects observed with lipApoE3/E4 and decreases the uptake of cholesteryl esters (CEs), which are lipids linked to neurodegeneration. In human neurons, the addition of ApoE carrying polyunsaturated fatty acids (PUFAs)-CE revealed an allelic series (ApoE4 > ApoE3 > ApoE2) associated with lipofuscinosis, an age-related lysosomal pathology resulting from lipid peroxidation. Lipofuscin increased lysosomal accumulation of tau fibrils and was elevated in the APOE4 mouse brain with exacerbation by tau pathology. Intrahippocampal injection of PUFA-CE-lipApoE4 was sufficient to induce lipofuscinosis in wild-type mice. Finally, the protective Christchurch mutation also reduced LDLR binding and phenocopied ApoE2. Collectively, our data strongly suggest decreased lipApoE-LDLR interactions minimize LOAD risk by reducing the deleterious effects of endolysosomal targeting of ApoE and associated pathogenic lipids.
中文翻译:

脂质化 ApoE 受体相互作用减少可保护 ApoE 及其溶酶体中的脂质货物免受致病性
虽然载脂蛋白 E (APOE) 是晚发性阿尔茨海默病 (LOAD) 的最强遗传修饰因子,但亚型依赖性风险的分子机制和 ApoE 相关脂质的相关性仍然难以捉摸。在这里,我们报道了脂质化 ApoE2 (lipApoE2) 的低密度脂蛋白 (LDL) 受体 (LDLR) 结合受损避免了 lipApoE3/E4 观察到的 LDLR 再循环缺陷,并减少了胆固醇酯 (CE) 的摄取,胆固醇酯 (CE) 是与神经退行性变相关的脂质。在人类神经元中,添加携带多不饱和脂肪酸 (PUFAs)-CE 的 ApoE 揭示了与脂褐质病相关的等位基因系列 (ApoE4 > ApoE3 > ApoE2),脂褐质变性是一种由脂质过氧化引起的年龄相关溶酶体病理。脂褐素增加了 tau 原纤维的溶酶体积累,并在 APOE4 小鼠脑中升高,并因 tau 病理而恶化。海马内注射 PUFA-CE-lipApoE4 足以诱导野生型小鼠脂肪褐质病。最后,保护性 Christchurch 突变也降低了 LDLR 结合和表型复制 ApoE2。总的来说,我们的数据强烈表明,减少 lipApoE-LDLR 相互作用通过减少内溶酶体靶向 ApoE 和相关致病性脂质的有害影响来最大限度地降低 LOAD 风险。
更新日期:2024-11-12
中文翻译:

脂质化 ApoE 受体相互作用减少可保护 ApoE 及其溶酶体中的脂质货物免受致病性
虽然载脂蛋白 E (APOE) 是晚发性阿尔茨海默病 (LOAD) 的最强遗传修饰因子,但亚型依赖性风险的分子机制和 ApoE 相关脂质的相关性仍然难以捉摸。在这里,我们报道了脂质化 ApoE2 (lipApoE2) 的低密度脂蛋白 (LDL) 受体 (LDLR) 结合受损避免了 lipApoE3/E4 观察到的 LDLR 再循环缺陷,并减少了胆固醇酯 (CE) 的摄取,胆固醇酯 (CE) 是与神经退行性变相关的脂质。在人类神经元中,添加携带多不饱和脂肪酸 (PUFAs)-CE 的 ApoE 揭示了与脂褐质病相关的等位基因系列 (ApoE4 > ApoE3 > ApoE2),脂褐质变性是一种由脂质过氧化引起的年龄相关溶酶体病理。脂褐素增加了 tau 原纤维的溶酶体积累,并在 APOE4 小鼠脑中升高,并因 tau 病理而恶化。海马内注射 PUFA-CE-lipApoE4 足以诱导野生型小鼠脂肪褐质病。最后,保护性 Christchurch 突变也降低了 LDLR 结合和表型复制 ApoE2。总的来说,我们的数据强烈表明,减少 lipApoE-LDLR 相互作用通过减少内溶酶体靶向 ApoE 和相关致病性脂质的有害影响来最大限度地降低 LOAD 风险。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号