Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-11-11 , DOI: 10.1002/adsc.202401322 Ai Zhang, Huangfeng Zhang, Tao Jin, Lin Ge, Xiaoyan Ma, Jinghua Tang, Jinyu Liu, Choon-Hong Tan, Richmond Lee, Yicen Ge
|
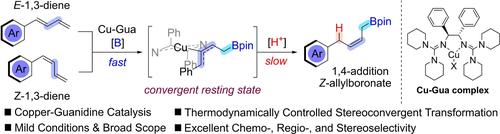
Merging the stereoisomeric mixture of substrate into a single product through stereospecific transformation is a challenging but higher-order synthetic strategy, which perfectly meets the demand of cost control in the precise chemical production. In this work, the stereoconvergent synthesis of Z-allylic boronates was realized with protoborylation of E/Z-mixed 1,3-dienes catalyzed by a novel copper-guanidine complex. The reaction could proceed smoothly under very mild conditions with good functional group tolerance, and convert diverse aryl-substituted 1,3-dienes into the desired Z-1,4-addition products with excellent chemo-, regio-, and stereoselectivities in minutes. Detailed mechanistic studies also helped to disclose the origin of stereoconvergency. Both E- and Z-diene were found directly undergoing a rapid borylation without E/Z isomerization of C=C bond, followed by a convergent formation of the same thermodynamically stable allylcopper intermediate before the slow protonation step occurred.
中文翻译:

通过 Cu-胍催化从 E/Z 混合 1,3-二烯中立体收敛获得 Z-烯丙基硼
通过立体特异性转化将底物的立体异构混合物合并成单一产物是一种具有挑战性但更高阶的合成策略,它完美地满足了精密化工生产中成本控制的需求。在这项工作中,通过新型铜-胍络合物催化的 E/Z 混合 1,3-二烯烃的原硼化实现了 Z-烯丙基硼酸盐的立体收敛合成。反应可以在非常温和的条件下顺利进行,具有良好的官能团耐受性,并在几分钟内将不同的芳基取代的 1,3-二烯转化为所需的 Z-1,4-加成产物,具有优异的化学选择性、区域选择性和立体选择性。详细的机制研究也有助于揭示立体收敛性的起源。发现 E-和 Z-二烯都直接经历快速硼化,而没有 C=C 键的 E/Z 异构化,随后在缓慢质子化步骤发生之前收敛形成相同的热力学稳定的烯丙基铜中间体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号