当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Superior Photostability of the Unnatural Base 6-Amino-5-nitropyridin-2-ol: A Case Study Using Ultrafast Broadband Fluorescence, Transient Absorption, and Theoretical Computation
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.jpclett.4c02751 Qingwu Xiong, Ping Wang, Chensheng Ma, Alvis Tsz-Kit Law, Mingliang Wang, Wai-Ming Kwok
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.jpclett.4c02751 Qingwu Xiong, Ping Wang, Chensheng Ma, Alvis Tsz-Kit Law, Mingliang Wang, Wai-Ming Kwok
|
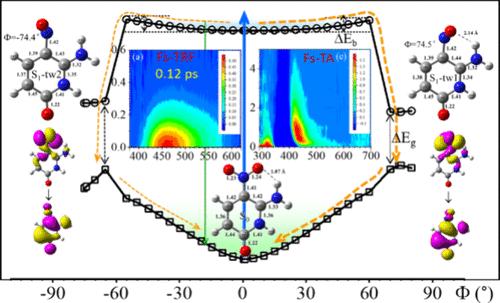
6-Amino-5-nitropyridin-2-ol (Z), a nitroaromatic compound and a base for Hachimoji nucleic acids, holds significant potential in expanding the genetic alphabet, as well as in synthetic biology and biotechnology. Despite its promising applications, the spectral characterization and photoinduced properties of Z have remained largely unexplored until now. This study presents a comprehensive investigation into its excited state dynamics in various solvents, utilizing state-of-the-art ultrafast broadband time-resolved fluorescence and transient absorption spectroscopy, complemented by computational methods. The acquired results provide direct experimental evidence that, upon photoexcitation, Z emits prompt fluorescence from a nearly planar structure in its excited state, independent of solvent properties. This state deactivates nonradiatively within sub-picoseconds through internal conversion with a unitary yield, primarily mediated by the rotation of the nitro group. This unusually rapid deactivation pathway entirely excludes the involvement of long-lived nπ* states, triplet states, and photoproducts, which are commonly observed in most nitroaromatic compounds and natural DNA and RNA bases. Our findings underscore that Z, as an unnatural base, exhibits superior photostability compared to canonical natural bases. This provides valuable insights into the photodynamics of nitroaromatic compounds, which is beneficial for strategic substitution design in environmental and biological applications.
中文翻译:

非天然碱基 6-氨基-5-硝基吡啶-2-醇的卓越光稳定性:使用超快宽带荧光、瞬态吸收和理论计算的案例研究
6-氨基-5-硝基吡啶-2-醇 (Z) 是一种硝基芳香族化合物,也是八目寺核酸的碱基,在扩展遗传字母表以及合成生物学和生物技术方面具有重要潜力。尽管 Z 的光谱表征和光诱导特性的应用前景广阔,但到目前为止,Z 的光谱表征和光诱导特性在很大程度上仍未得到探索。本研究利用最先进的超快宽带时间分辨荧光和瞬态吸收光谱,并辅以计算方法,对其在各种溶剂中的激发态动力学进行了全面研究。获得的结果提供了直接的实验证据,即在光激发时,Z 从处于激发态的近平面结构中发出快速荧光,与溶剂性质无关。这种状态在亚皮秒内通过内部转换以幺正产率非辐射性地失活,主要由硝基的旋转介导。这种异常快速的失活途径完全排除了长寿命 nπ* 态、三重态和光产物的参与,这些在大多数硝基芳香族化合物和天然 DNA 和 RNA 碱基中很常见。我们的研究结果强调,Z 作为一种非天然碱基,与经典的天然碱基相比,表现出优异的光稳定性。这为硝基芳香族化合物的光动力学提供了有价值的见解,有利于环境和生物应用中的战略取代设计。
更新日期:2024-11-12
中文翻译:

非天然碱基 6-氨基-5-硝基吡啶-2-醇的卓越光稳定性:使用超快宽带荧光、瞬态吸收和理论计算的案例研究
6-氨基-5-硝基吡啶-2-醇 (Z) 是一种硝基芳香族化合物,也是八目寺核酸的碱基,在扩展遗传字母表以及合成生物学和生物技术方面具有重要潜力。尽管 Z 的光谱表征和光诱导特性的应用前景广阔,但到目前为止,Z 的光谱表征和光诱导特性在很大程度上仍未得到探索。本研究利用最先进的超快宽带时间分辨荧光和瞬态吸收光谱,并辅以计算方法,对其在各种溶剂中的激发态动力学进行了全面研究。获得的结果提供了直接的实验证据,即在光激发时,Z 从处于激发态的近平面结构中发出快速荧光,与溶剂性质无关。这种状态在亚皮秒内通过内部转换以幺正产率非辐射性地失活,主要由硝基的旋转介导。这种异常快速的失活途径完全排除了长寿命 nπ* 态、三重态和光产物的参与,这些在大多数硝基芳香族化合物和天然 DNA 和 RNA 碱基中很常见。我们的研究结果强调,Z 作为一种非天然碱基,与经典的天然碱基相比,表现出优异的光稳定性。这为硝基芳香族化合物的光动力学提供了有价值的见解,有利于环境和生物应用中的战略取代设计。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号